MEDIUM
JEE Main
IMPORTANT
Earn 100
Which of the following is an equivalent cyclic process corresponding to the thermodynamic cyclic given in the figure? Where, is adiabatic. (Graphs are schematic and are not to scale)
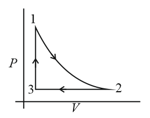
(a)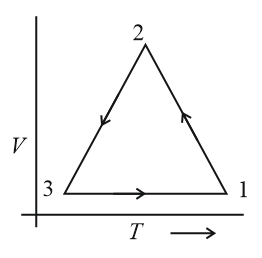

(b)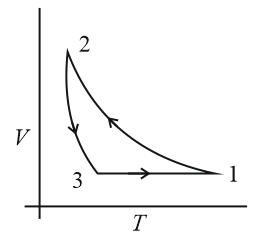

(c)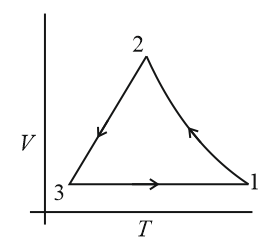

(d)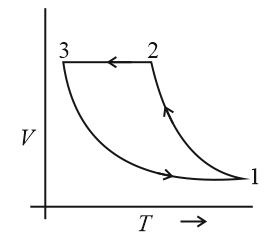

47.82% studentsanswered this correctly

Important Questions on Thermodynamics
MEDIUM
JEE Main
IMPORTANT
Starting at temperature one mole of an ideal diatomic gas is first compressed adiabatically from volume to It is then allowed to expand isobarically to volume . If all the processes are the quasi-static then the final temperature of the gas (in ) is (to the nearest integer) ___________.
EASY
JEE Main
IMPORTANT
Following figure shows two processes and for a gas. If and are the amount of heat absorbed by the system in two cases, and and are changes in internal energies, respectively, then:
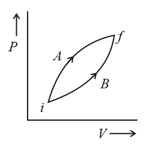
HARD
JEE Main
IMPORTANT
The specific heats, and of a gas of diatomic molecules, are given (in units of ) by and respectively. Another gas of diatomic molecules, has the corresponding values and If they are treated as ideal gases, then:
EASY
JEE Main
IMPORTANT
A gas can be taken from to via two different processes and .
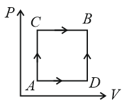
When path is used of heat flows into the system and of work is done by the system. If the path is used then work done by the system is , the heat flows into the system in the path is:
EASY
JEE Main
IMPORTANT
Two carnot engines and are operated in series. The first one, receives heat at and rejects to a reservoir at temperature The second engine receives heat rejected by the first engine and, in turn, rejects to a heat reservoir at Calculate the temperature if the work outputs of the two engines are equal:
EASY
JEE Main
IMPORTANT
The given diagram shows four processes i.e., isochoric, isobaric, isothermal and adiabatic. The correct assignment of the processes, in the same order is given by:
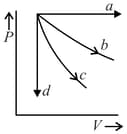
EASY
JEE Main
IMPORTANT
moles of an ideal gas with constant volume heat capacity undergo an isobaric expansion by certain volume. The ratio of the work done in the process, to the heat supplied is:
MEDIUM
JEE Main
IMPORTANT
A cylinder with fixed capacity of litre contains helium gas at STP. The amount of heat needed to raise the temperature of the gas by is:
[Given that
