EASY
Earn 100
Which of the following is incorrect regarding salt bridge solution?
(a)Solution must be a strong electrolyte
(b)Solution should be inert towards both electrodes
(c)Size of cations and anions of salt should be much different
(d)Salt bridge solution is prepared in gelatin or agar-agar to make it semi-solid
50% studentsanswered this correctly
Important Questions on Electrochemistry
EASY
HARD
MEDIUM
MEDIUM
EASY
At , the of the galvanic cell mentioned below is
MEDIUM
Represent a cell consisting of | half cell and | half cell and write the cell reaction.
HARD
EASY
EASY
MEDIUM
The following reaction takes place at in an electrochemical cell involving two metals and ,
with and in the respective half-cells, the cell EMF is . The equilibrium constant of the reaction is closest to;
MEDIUM
(a)
(b)
EASY
,
HARD
What are Galvanic cells? Explain the working of Galvanic cells with one example.
MEDIUM
EASY
HARD
The reaction occurs in which of the given galvanic cell?
MEDIUM
EASY
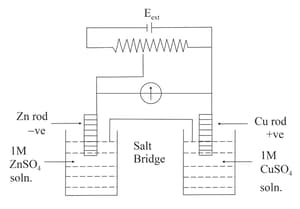
Identify the incorrect statement from the options below for the above cell:
HARD
For the cell , when the concentration of is times the concentration of , the expression for is
Faraday's constant, universal gas constant, temperature,
HARD

