EASY
JEE Main/Advance
IMPORTANT
Earn 100
Which of the following is not a state function?
(a)
(b)
(c)enthalpy
(d)entropy.
42.86% studentsanswered this correctly

Important Questions on Thermodynamics
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
A coffee cup calorimeter initially contains of water, at a temperature of . of ammonium nitrate (NH4NO3), also at , is added to the water, and the final temperature is . What is the heat of solution of ammonium nitrate in kJ/mol? The specific heat capacity of the solution is .
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
When mole of ice melts at at constant pressure of atm, calories of heat are absorbed by the system. Calculate the for the reaction.
HARD
JEE Main/Advance
IMPORTANT
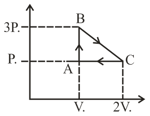
One mole of ideal monoatomic gas is carried through the reversible cyclic process as shown in figure. Calculate the net heat absorbed by the gas in the path BC.
HARD
JEE Main/Advance
IMPORTANT
The enthalpy of combustion of propane gas in terms of given data is :
Bond energy
Resonance energy of is and is .
