EASY
10th CBSE
IMPORTANT
Earn 100
Which of the following is not a thermal decomposition reaction?
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Chemical Reactions And Equations
EASY
10th CBSE
IMPORTANT
MEDIUM
10th CBSE
IMPORTANT
MEDIUM
10th CBSE
IMPORTANT
MEDIUM
10th CBSE
IMPORTANT
EASY
10th CBSE
IMPORTANT
MEDIUM
10th CBSE
IMPORTANT
EASY
10th CBSE
IMPORTANT
Complete the chemical reaction.
MEDIUM
10th CBSE
IMPORTANT
Study the given diagram carefully.
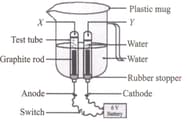
Identify X, Y and the type of reaction occurring.
