EASY
Earn 100
Which of the following is not correct in case of kinetic theory of gases?
(a)Gases are made up of small particles of neglible size as compared to container size
(b)The molecules are in random motion always
(c)When molecules Collide they lose energy
(d)When the gas is heated, the average kinetic energy of gas molecules increase,
50% studentsanswered this correctly
Important Questions on States of Matter
EASY
MEDIUM
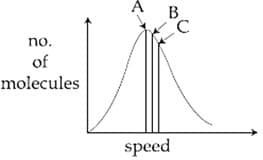
Root mean square speed most proable speed Average speed
EASY
MEDIUM
EASY
MEDIUM
HARD
If the distribution of molecular speeds of a gas is as per the figure shown below, then the ratio of the most probable, the average, and the root mean square speeds, respectively, is
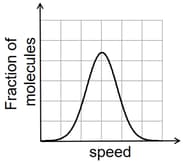
MEDIUM
MEDIUM
EASY
MEDIUM
MEDIUM
EASY
MEDIUM
EASY
EASY
EASY
MEDIUM
EASY
EASY

