HARD
JEE Main
IMPORTANT
Earn 100
Which of the following is the correct plot for the probability density as a function of distance of the electron form the nucleus for orbital?
(a)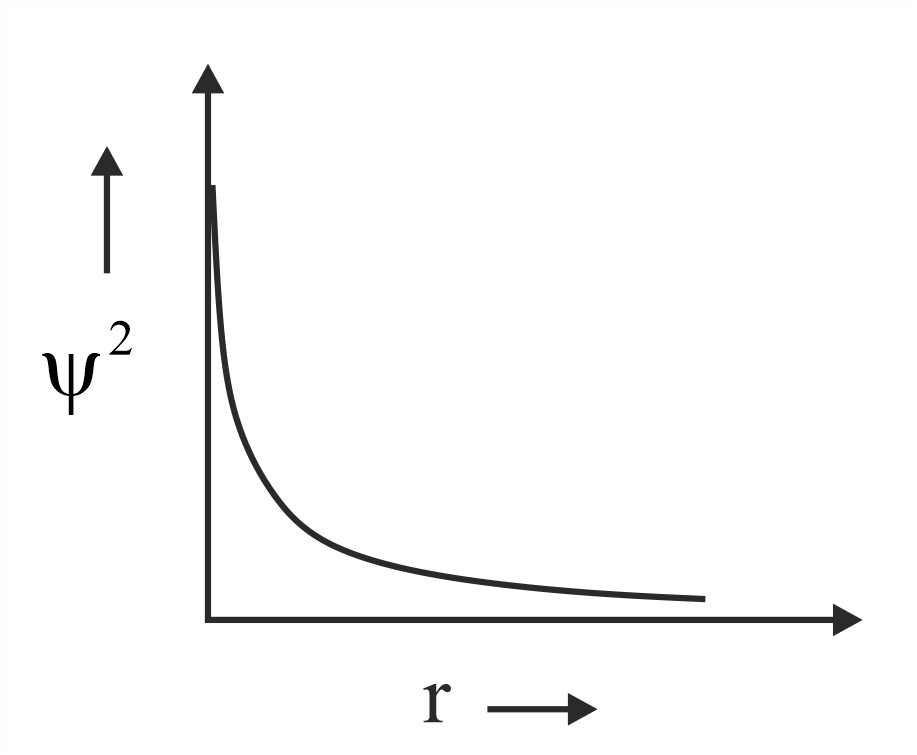

(b)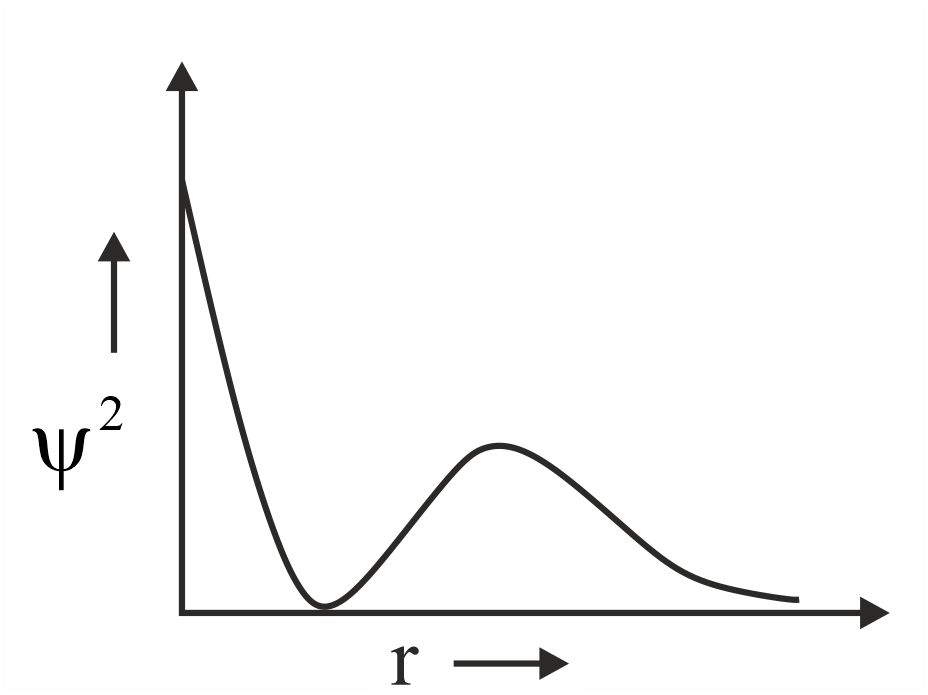

(c)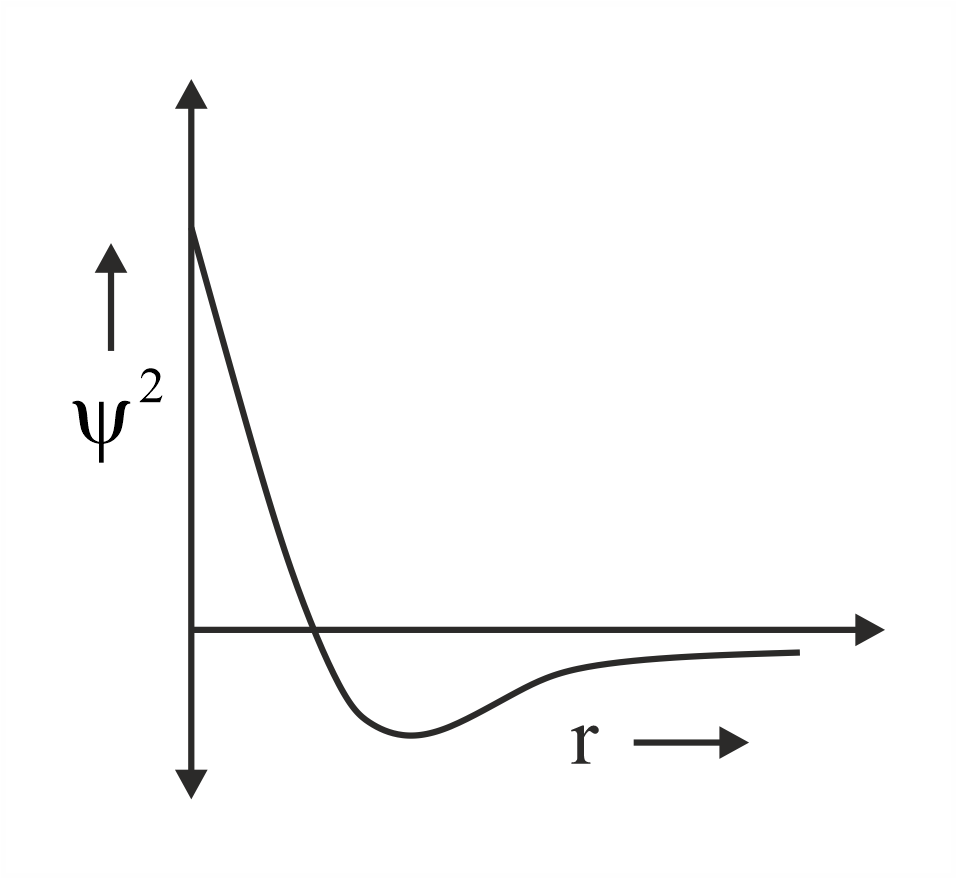

(d)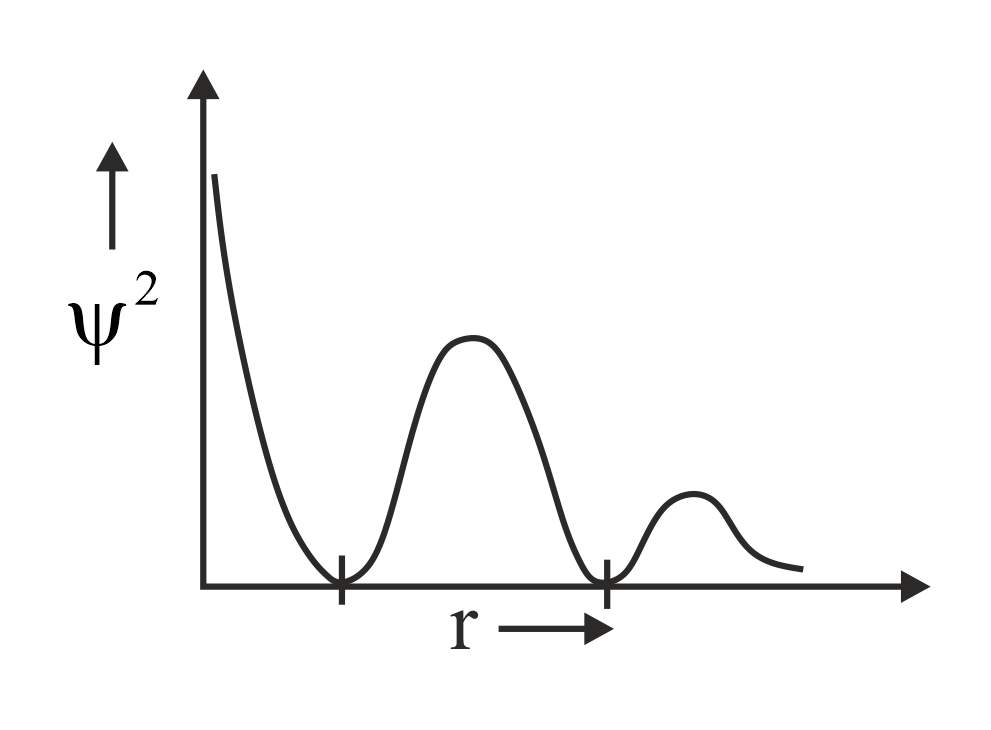

80.49% studentsanswered this correctly

Important Questions on Structure of Atom
MEDIUM
JEE Main
IMPORTANT
Which of the following sets of quantum numbers is not allowed?
MEDIUM
JEE Main
IMPORTANT
When the excited electron of a atom from drops to the ground state, the maximum number of emission lines observed are
HARD
JEE Main
IMPORTANT
The wavelength of an electron and a neutron will become equal when the velocity of the electron is times the velocity of neutron. The value of is____(the nearest integer)(Mass of electron is and mass of neutron is )
HARD
JEE Main
IMPORTANT
Given below are two statements. One is labelled as Assertion and the other is labelled as Reason .
Assertion : Energy of orbital of hydrogen atom is greater than that of orbital of lithium.
Reason : Energies of the orbitals in the same subshell decrease with increase in the atomic number.
In the light of the above statements, choose the correct answer from the options given below.
EASY
JEE Main
IMPORTANT
The correct decreasing order of energy, for the orbitals having, following set of quantum numbers:
(A)
(B)
(C)
(D)
MEDIUM
JEE Main
IMPORTANT
Identify the incorrect statement from the following.
EASY
JEE Main
IMPORTANT
Which of the following pair is not isoelectronic species?
(Atomic numbers )
HARD
JEE Main
IMPORTANT
If the wavelength of an electron emitted from atom is , then energy absorbed by the electron in its ground state compared to the minimum energy required for its escape from the atom, is____times.
[Given : , Mass of electron ], Give an answer to the nearest integer value.
