Which of the following metals shows the fastest reaction with cold water?

Important Questions on Reactivity and Rates of Reaction
A teacher carefully cut some samples of metals and left them in the air. The class timed how long it took for the cut edge of the metals to go dull and recorded the results on scrap paper. The times were:
lithium: , potassium: , calcium: , sodium
- Put the data into a table. Start by giving each column a heading.
A teacher carefully cut some samples of metals and left them in the air. The class timed how long it took for the cut edge of the metals to go dull and recorded the results on scrap paper. The times were:
lithium: , potassium: , calcium: , sodium
- Underline the metal showing the fastest reaction.
A teacher carefully cut some samples of metals and left them in the air. The class timed how long it took for the cut edge of the metals to go dull and recorded the results on scrap paper. The times were:
lithium: , potassium: , calcium: , sodium
- Underline the metal showing the slowest reaction.
Balance the symbol equation for the reaction of magnesium with oxygen.
Balance the symbol equation for the reaction of magnesium with oxygen.
Draw the atom on each side of the equation.
Start by trying to balance the hydrogen atoms. Multiply all the atoms in _____ by .
To balance the oxygen atoms, multiply all the atoms in _____ by .
To balance the sodium atoms, multiply the sodium atoms on the _____ by .
The numbers of atoms on each side of the equation are the same. Go back to the equation at the start of the question and write in the multipliers in front of the atoms and compounds.
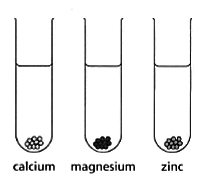
When calcium reacts with water, the reaction is:
- More vigorous (faster) than the reaction between zinc and water.
- Less vigorous (slower) than the reaction between magnesium and water.
Predict how the metals will react in hydrochloric acid by drawing bubbles in the test tubes.
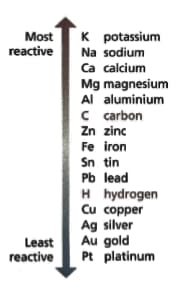
Aluminium is above iron in the reactivity series of metals.
Observations show that objects made from aluminium do not corrode as quickly as those made from iron. Use your research to explain why aluminium corrodes less quickly than expected to explain the observations.
Look at the diagram of the reactivity series — it shows the metals in order of reactivity. Read these statements:
- Copper beads over years old have been found in Northern Iraq.
- The ancient Hittites of Asia Minor extracted iron in the .
- Sodium metal was first extracted in .
Describe the pattern you can see between a metal’s place in the reactivity series and its discovery.
