MEDIUM
JEE Main
IMPORTANT
Earn 100
Which of the following molecules can exhibit optical activity?
(a)-bromopropane
(b)-bromobutane
(c)-bromopentane
(d)bromocyclohexane
50% studentsanswered this correctly

Important Questions on Organic Chemistry- Some Basic Principles and Techniques
MEDIUM
JEE Main
IMPORTANT
What is the structure of an optical active compound which decolourises bromine water.
MEDIUM
JEE Main
IMPORTANT
An organic compound fused with metallic sodium dissolves in water and the solution is divided into two parts, one part is treated with , boiled and filtered. In the filtrate, addition of does not produce any precipitate. To the other part, addition of sodium nitro prusside produces violet colour. The organic compound contains.
MEDIUM
JEE Main
IMPORTANT
The decreasing order of the rate of the above reaction with nucleophiles is
MEDIUM
JEE Main
IMPORTANT
The ammonia evolved from of a compound in Kjeldahl's estimation of nitrogen neutralizes of solution. The weight percentage of nitrogen in the compound is:
MEDIUM
JEE Main
IMPORTANT
Consider the following organic compound:
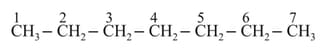
To make it a chiral compound, the attack should be on carbon atom number.
MEDIUM
JEE Main
IMPORTANT
Which of the following is not true for maleic acid and fumaric acid.
MEDIUM
JEE Main
IMPORTANT
The number of optically active stereoisomers possible for butane- -diol.
MEDIUM
JEE Main
IMPORTANT
Among , and , the weakest acid in water is:
