HARD
NEET
IMPORTANT
Earn 100
Which of the following curve represents maximum work done?
(a)
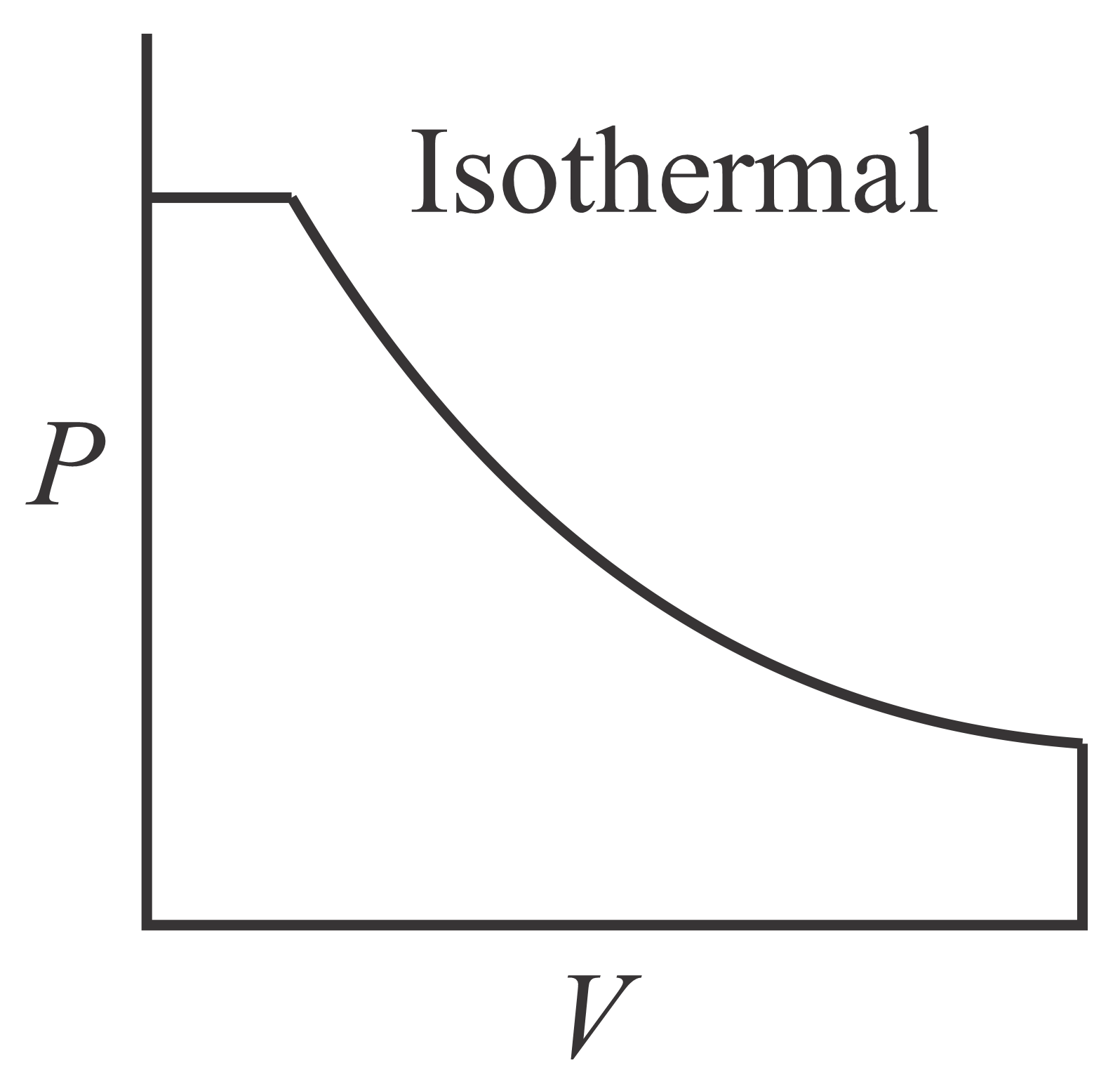
(b)
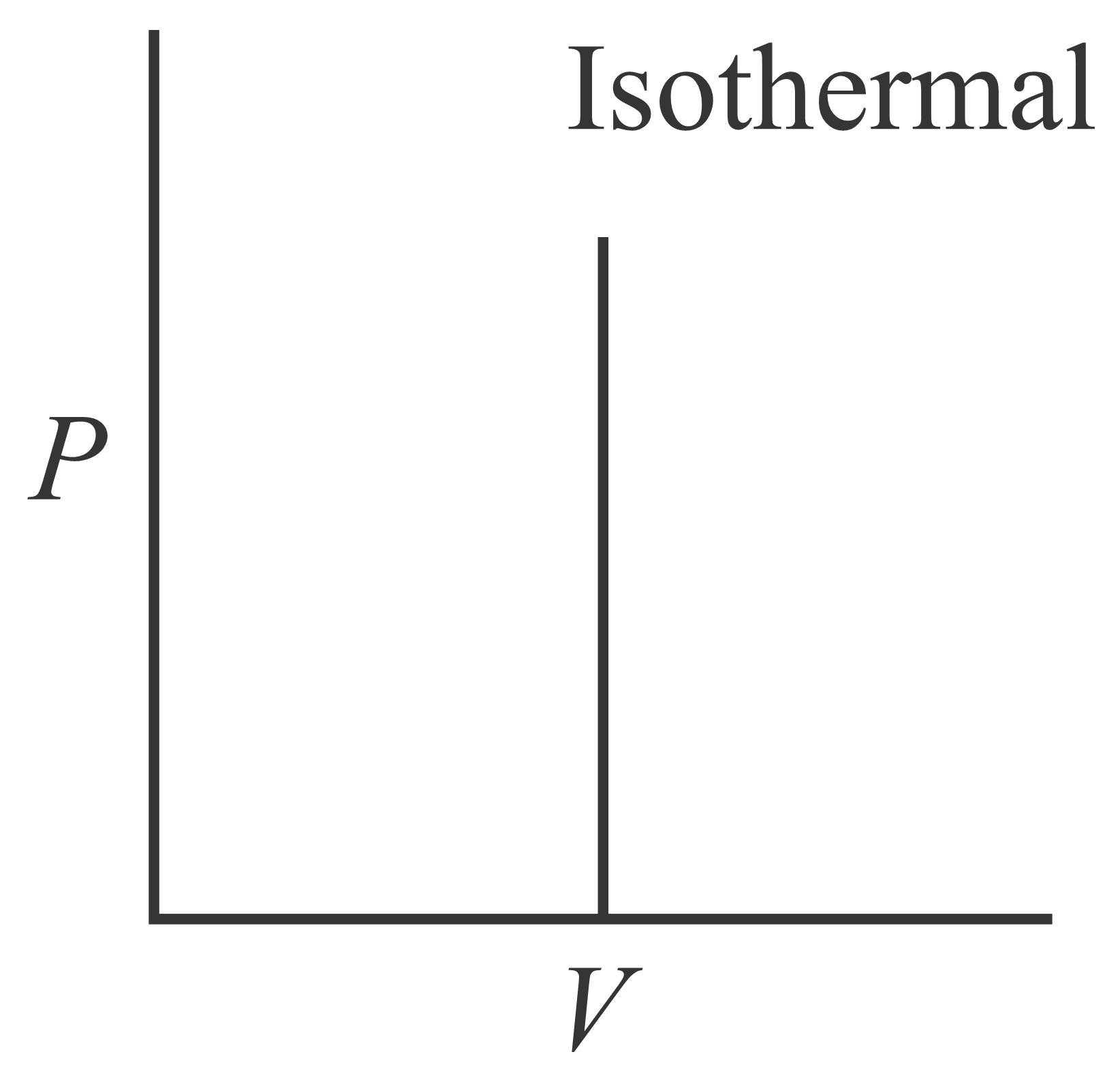
(c)
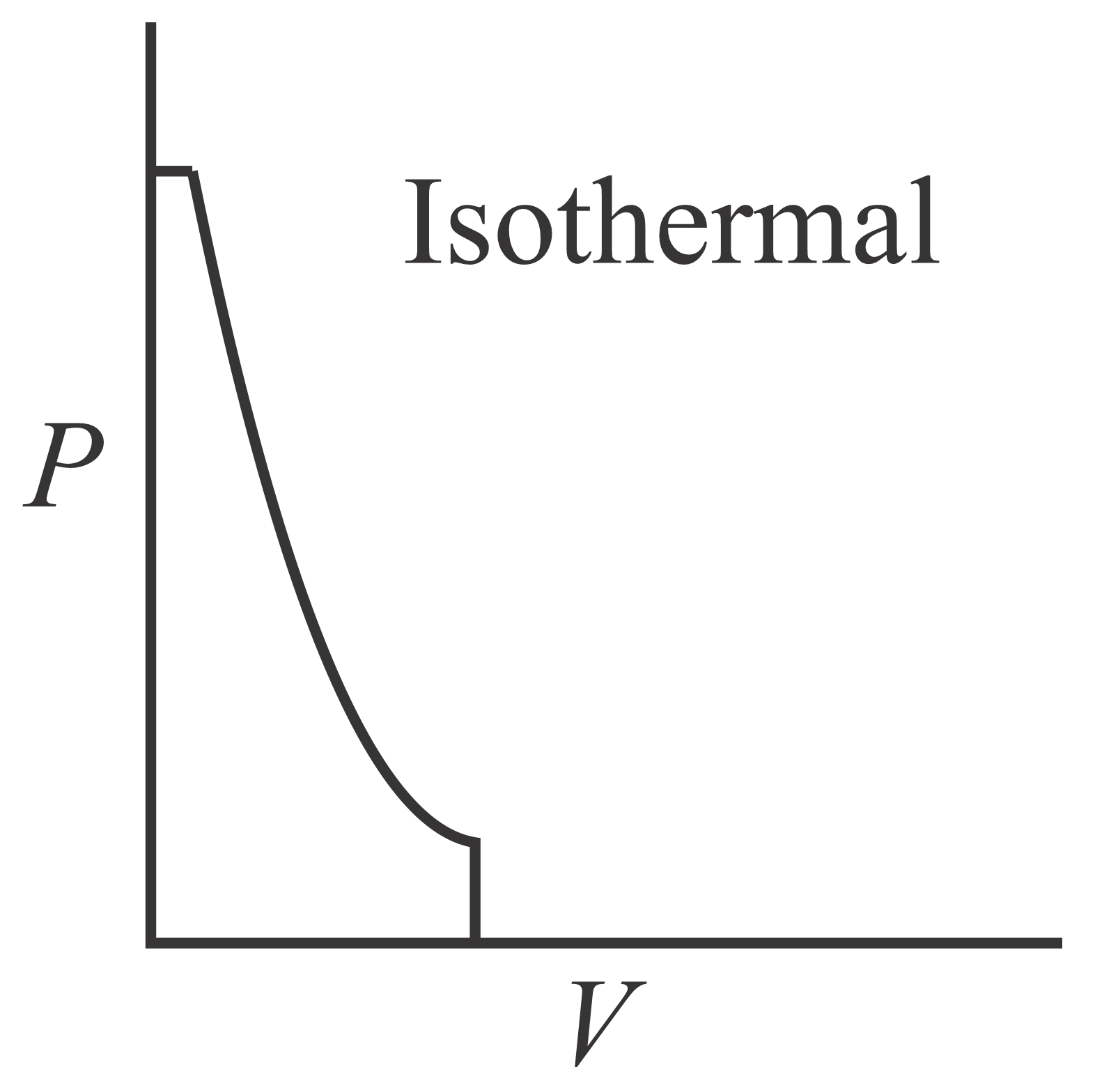
(d)
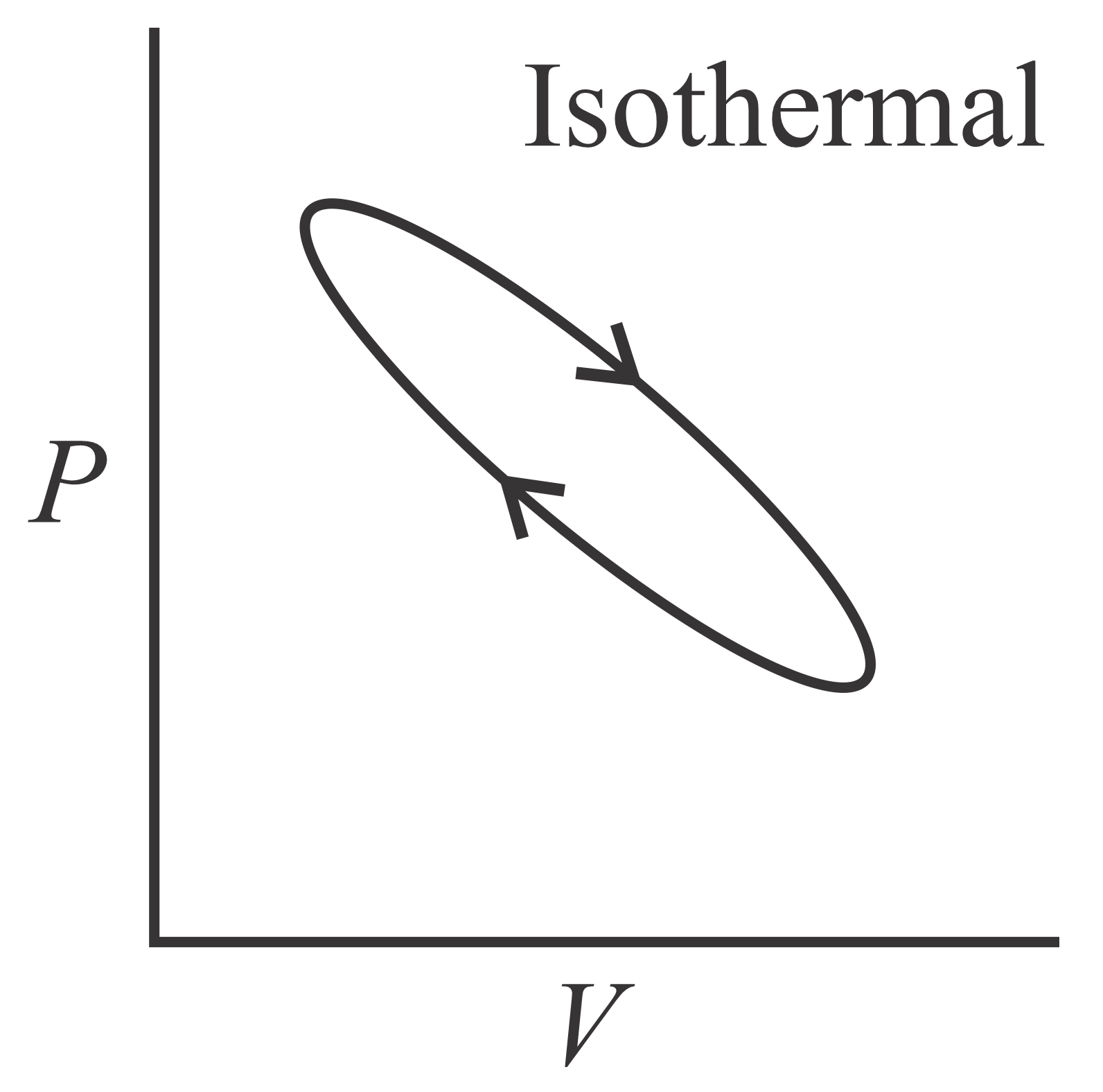
90.91% studentsanswered this correctly

Important Questions on Thermodynamics
EASY
NEET
IMPORTANT
Which one among the following is the correct option for right relationship between and for one mole of ideal gas?
EASY
NEET
IMPORTANT
For irreversible expansion of an ideal gas under isothermal condition, the correct option is:
MEDIUM
NEET
IMPORTANT
If for a certain reaction is at the value of (in ) for which the same reaction will be spontaneous at the same temperature is
MEDIUM
NEET
IMPORTANT
At standard conditions, if the change in the enthalpy for the following reaction is
Given that bond energy of and is and , respectively, what is the bond energy (in ) of
EASY
NEET
IMPORTANT
For the reaction, , the correct option is
MEDIUM
NEET
IMPORTANT
The correct option for free expansion of an ideal gas under adiabatic condition is
EASY
NEET
IMPORTANT
Under the isothermal condition, a gas at expands from to against a constant external pressure of bar. The work done by the gas is
(Given that bar)
EASY
NEET
IMPORTANT
In which case change in entropy is negative?
