MEDIUM
Earn 100
Which of the following parameters would be expected to have the same values for the structural isomers and ? (Assume ideal behaviour)
(a)Boiling points
(b)Vapour pressure at the same temperature
(c)Heat of vaporization
(d)Gaseous densities at the same temperature and pressure
50% studentsanswered this correctly
Important Questions on Basic Principles of Organic Chemistry
MEDIUM
MEDIUM
MEDIUM
MEDIUM
Which of the following carbonyl compounds will exhibit enolization?
(i) 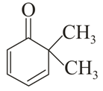
(ii)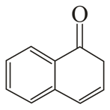
(iii) 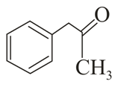
(iv) 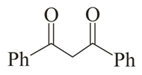
(v) 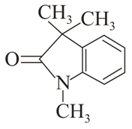
MEDIUM
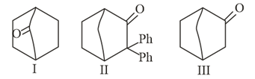
MEDIUM
HARD
MEDIUM
Assertion A : Enol form of acetone exists in quantity. However, the enol form of acetyl acetone exists in approximately quantity.
Reason R : enol form of acetyl acetone is stabilized by intramolecular hydrogen bonding, which is not possible in enol form of acetone.
Choose the correct statement:
EASY
EASY
MEDIUM
MEDIUM
Given,
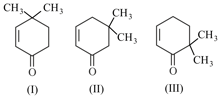
Which of the given compounds can exhibit tautomerism?
EASY
MEDIUM
EASY
The following compounds

are
EASY
EASY
MEDIUM
HARD
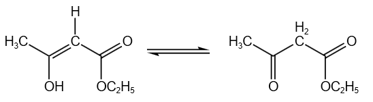
EASY

