MEDIUM
JEE Main
IMPORTANT
Earn 100
Which of the following plots is/are correct?
(a)
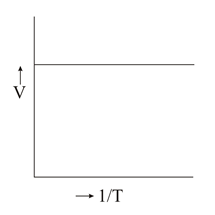
(b)
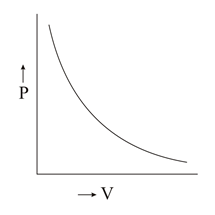
(c)
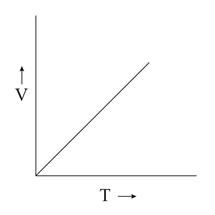
(d)
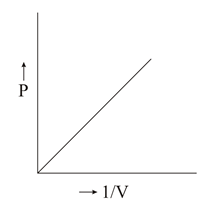
50% studentsanswered this correctly

Important Questions on States of Matter
MEDIUM
JEE Main
IMPORTANT
What should be the percentage increase in pressure for a decrease in volume of a gas at constant temperature?
HARD
JEE Main
IMPORTANT
The pressure exerted by of an ideal gas at temperature in a vessel of volume litre is one . When the temperature is increased by degree at the same volume, the pressure increases by . Calculate the temperature and volume . (Molar mass of the gas).
HARD
JEE Main
IMPORTANT
of a boron-hydrogen compound created a pressure of in a bulb of at . Analysis showed it to be boron. Calculate its molecular formula.
MEDIUM
JEE Main
IMPORTANT
Calculate the of 'free volume' available in gaseous water at and . Density of liquid water at is .
HARD
JEE Main
IMPORTANT
A mixture of and is placed in a sealed container at . The pressure within the container is . The container is heated to where, the two gases undergo decomposition reactions.
and
The pressure of the container at this stage become . What was the mole percent of in the original mixture?
MEDIUM
JEE Main
IMPORTANT
At , the density of a fixed mass of an ideal gas divided by its pressure is . At , this ratio would be:
HARD
JEE Main
IMPORTANT
Which of these gases exhibits behavior that deviates most significantly from that expected from an ideal gas?
HARD
JEE Main
IMPORTANT
Ten moles of an ideal gas is filled in a closed vessel. The vessel has cylinder and piston type arrangement and pressure of the gas remains constant at . Which of the following graph represents the correct variation of vs ? (Volume in litre and Temperature in kelvin)
