HARD
JEE Advanced
IMPORTANT
Earn 100
Which of the following reactions will give identical products?
(a)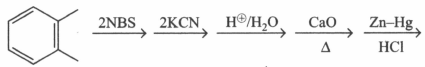
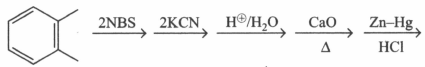
(b)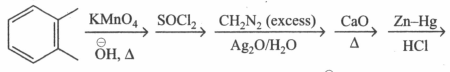
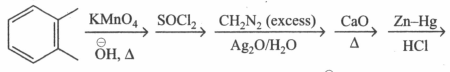
(c)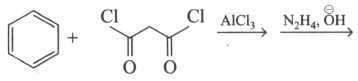

(d)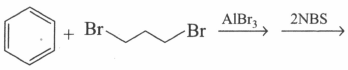

50% studentsanswered this correctly

Important Questions on Aromatic Compounds and Alkyl and Aryl Halides
HARD
JEE Advanced
IMPORTANT
Select the reactions in which the correct orientations have been mentioned in the major products.
HARD
JEE Advanced
IMPORTANT
On electrolysis an aqueous ethanolic solution of sodium chloride gives sweet smelling liquid . gives isocyanide test and condenses with acetone to form hypotonic. What is ?
HARD
JEE Advanced
IMPORTANT
One mole of a hydrocarbon reacts with mole of bromine, giving a dibromo compound . , on treatment with cold dilute alkaline, solution forms a compound . On ozonolysis, gives equimolar quantities of propanone and ethanal. Deduce the structure of .
MEDIUM
JEE Advanced
IMPORTANT
Explain the following :
reacts with to give alkyl cyanide, while results in isocyanide as the major product.
MEDIUM
JEE Advanced
IMPORTANT
Explain the following :
() Iodoform gives precipitate with on heating while does not.
MEDIUM
JEE Advanced
IMPORTANT
Explain the following:
Hydrogen atom of chloroform is definitely acidic, but that of methane is not.
MEDIUM
JEE Advanced
IMPORTANT
Explain the following:
() small amount of alcohol is added to chloroform bottles
MEDIUM
JEE Advanced
IMPORTANT
Explain the following:
reacts more rapidly with strong base in comparison to .
