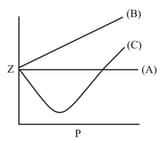
Which of the following represents a plot of compressibility factor (Z) versus P at room temperature for helium?
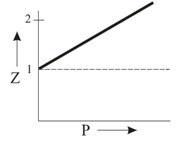
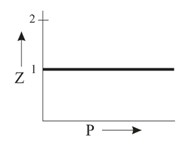
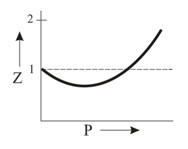
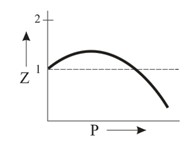
Important Questions on States of Matter: Gases and Liquids
A gas has a compressibility factor of and a molar volume of at a temperature of and pressure . If it shows ideal gas behaviour at the same temperature and pressure, the molar volume will be . The value of is _________
[Use: Gas constant, ]

Among the gases and , the gases that show only positive deviation from ideal behavior at all pressures in the graph are
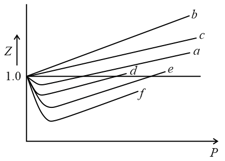
The volume of gas is twice than that of gas . The compressibility factor of gas is thrice than that of gas at same temperature. What are the pressures of the gases for equal number of moles?
The variation of the compressibility factorwith pressure for some gases, are shown in the figure below. Identify the gases and .
The isotherms of a gas are shown below :
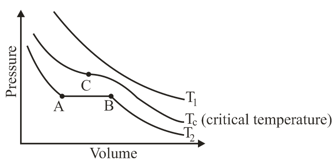
Among the following,
(i) At , the gas cannot be liquefied
(ii) At point , liquid starts to appear at
(ii) is the highest temperature at which the gas can be liquefied
(iv) At point , a small increase in pressure condenses the whole system to a liquid
The correct statements are :
[Given : " a " and " b " are standard parameters for van der Waals' gas]

