EASY
Earn 100
Which of the following represents the unit of the Boltzmann constant?
(a)
(b)
(c)
(d)
100% studentsanswered this correctly
Important Questions on Kinetic Theory of Gases and Radiation
EASY
MEDIUM
[Take gas constant as
HARD
Calculate the amount of each reactant required to produce of carbon dioxide, when two volumes of carbon monoxide combine with one volume of oxygen to produce two volumes of carbon dioxide.
State the law associated with this question.
EASY
EASY
HARD
If of gas A contains molecules, how many molecules of gas will be present in of ? The gases and are under the same conditions of temperature and pressure. Name the law on which the problem is based.
EASY
MEDIUM
MEDIUM
EASY
EASY
“The volume of gas molecules is taken into consideration in Avogadro’s Law.”
EASY
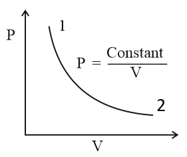
Out of the following which one correctly represents the diagram?
EASY
HARD
EASY
EASY
EASY
MEDIUM
HARD
(Atmospheric pressure = of Hg)
EASY

