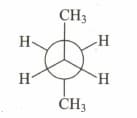
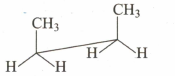
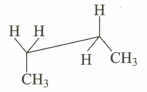
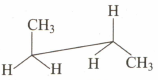
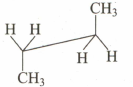
Which of the following sawhorse representation is correct for the given Newman projection?


Important Questions on Isomerism
The dihedral angle between two methyl groups in partially eclipsed conformation of -butane is
Consider the Newman projection formula of the most stable conformation of -hydroxypropanal. It is stable due to:
Which of the following is an achiral molecule?
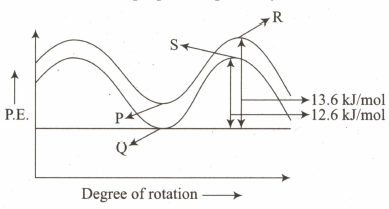
Which points on the potential energy diagram represent the eclipsed conformation of ethane and staggered conformation of propane respectively?
Amongst the given options, the compound(s) in which all the atoms are in one plane in all the possible conformations (if any), is (are):
Consider the following conformations of Aminopropanal.
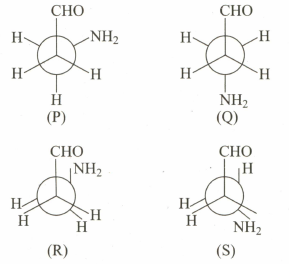
Amongst the above conformations () one of them is most stable.
This can be attributed due to-
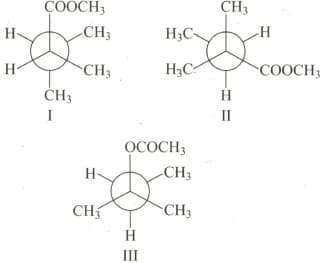
Correct relation between the above compounds:
