EASY
JEE Main
IMPORTANT
Earn 100
Which of the following shows the correct relationship between the pressure and density of an ideal gas at constant temperature ?
(a)
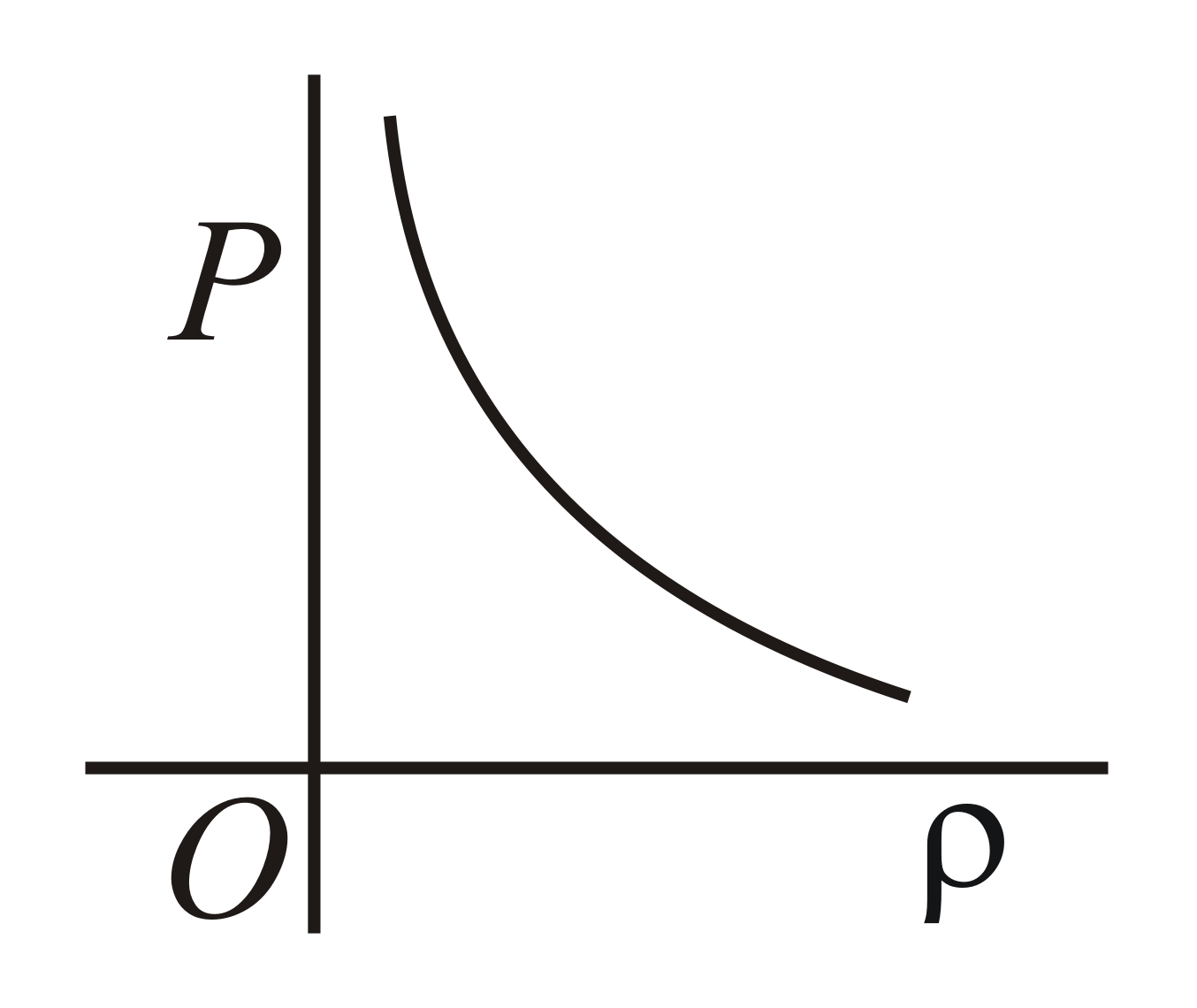
(b)
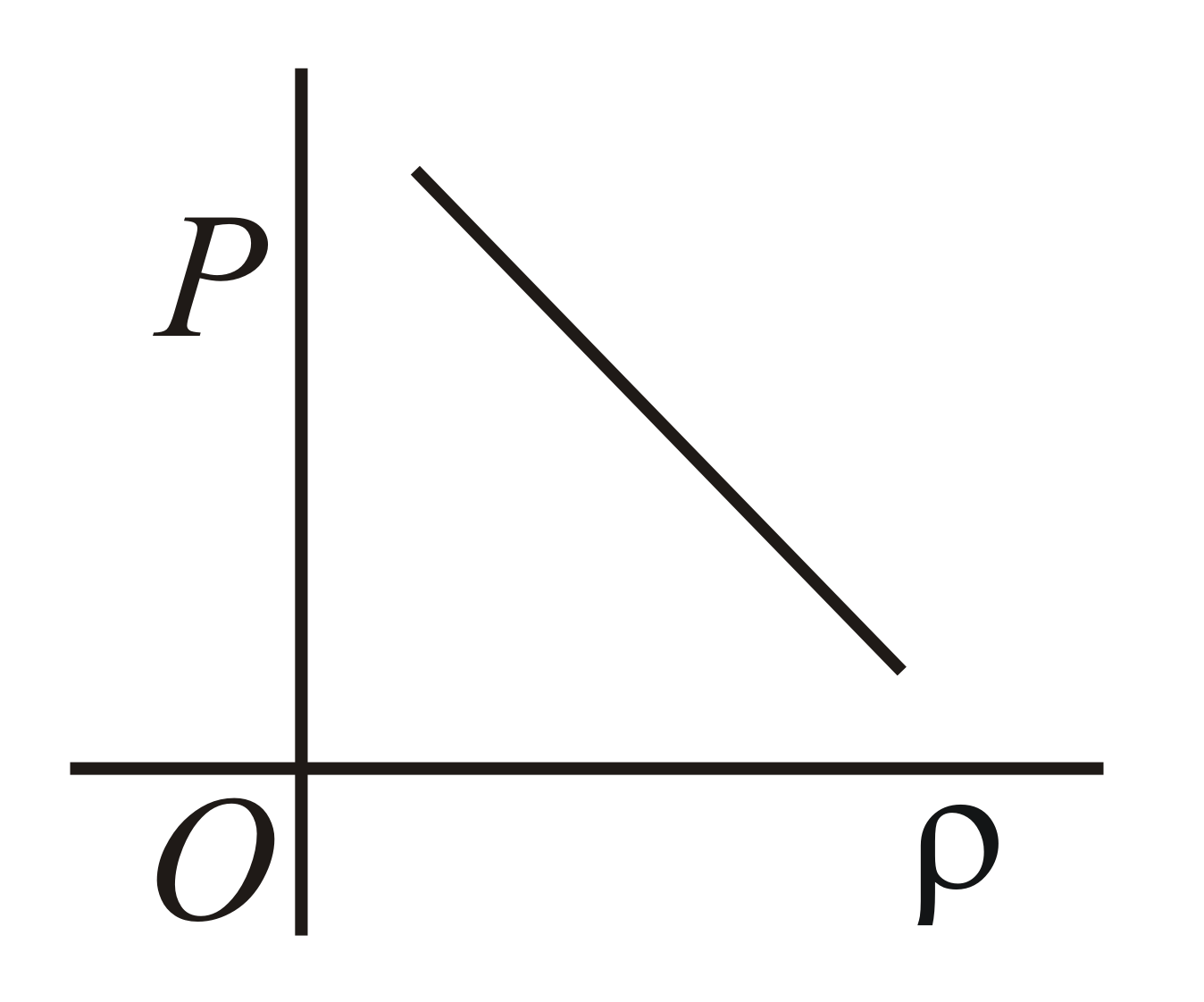
(c)
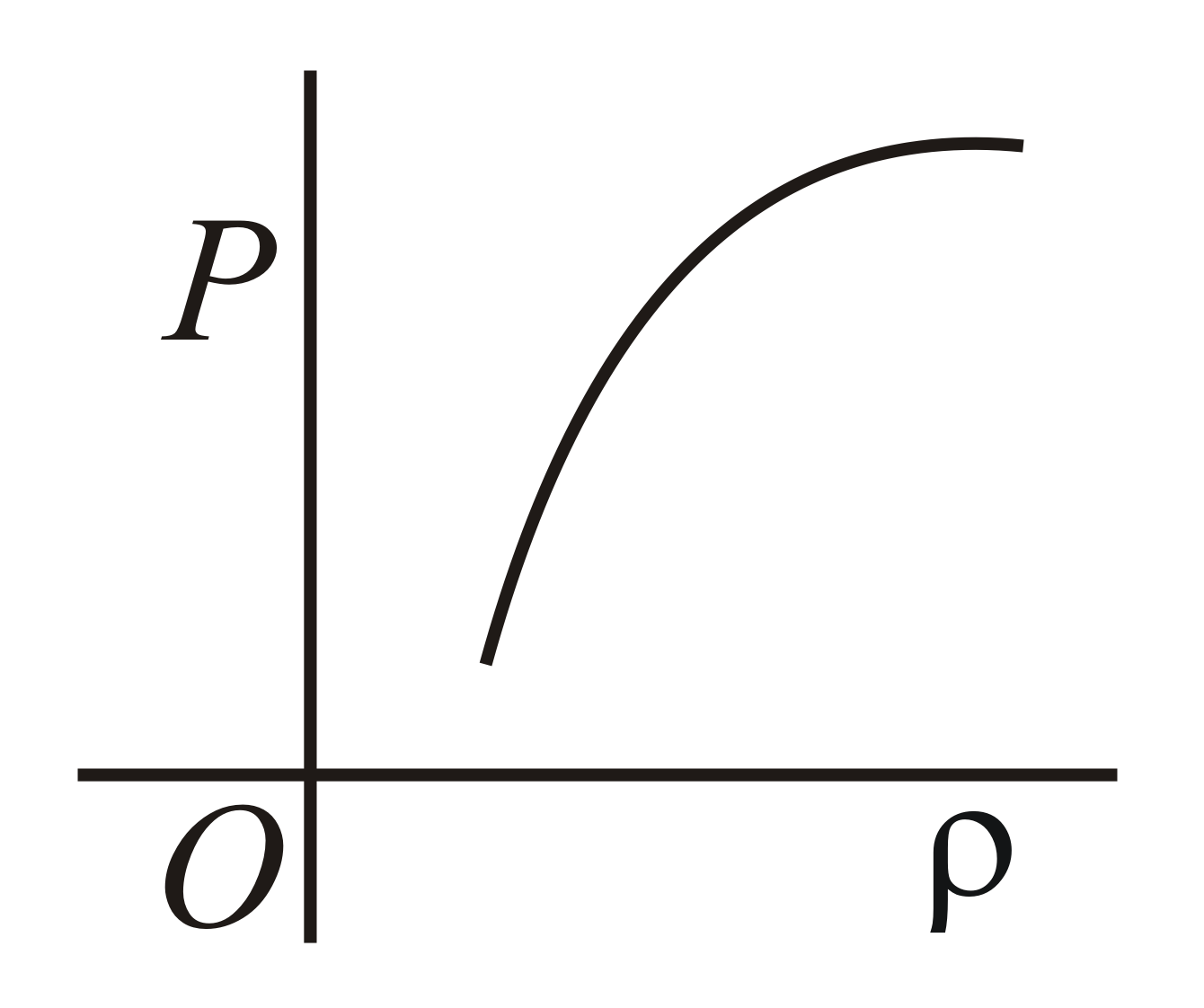
(d)
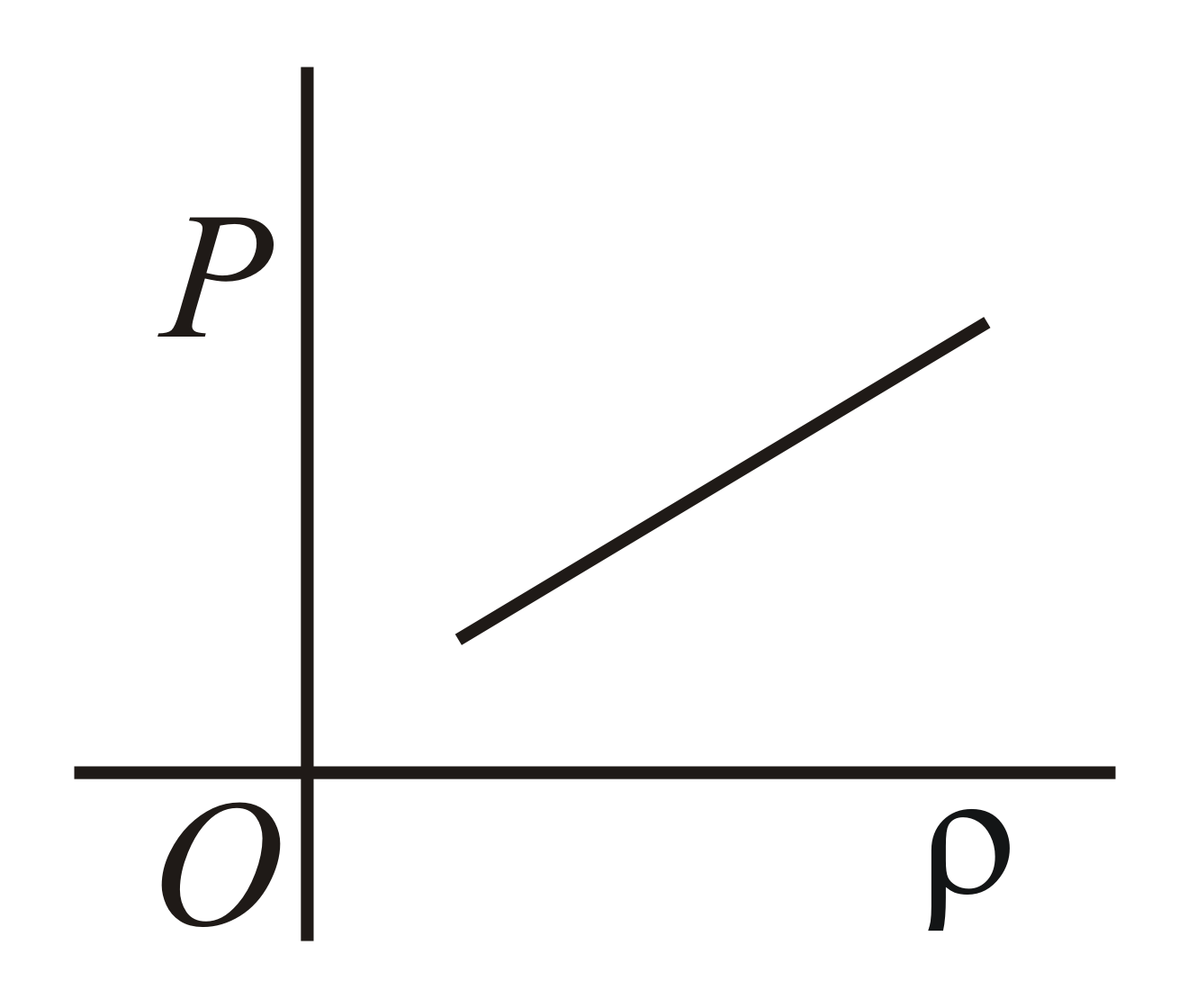
45.45% studentsanswered this correctly

Important Questions on Kinetic Theory of Gases
MEDIUM
JEE Main
IMPORTANT
In an ideal gas at temperature T, the average force that a molecule applies on the walls of a closed container depends on . A good estimate for q is:
HARD
JEE Main
IMPORTANT
Consider an ideal gas confined in an isolated closed chamber. As the gas undergoes an adiabatic expansion, the average time of collision between molecules increases as , where is the volume of the gas. The value of is:
HARD
JEE Main
IMPORTANT
Using equipartition of energy, the specific heat (in ) of Aluminium at high temperature can be estimated to be (atomic weight of Aluminium )
HARD
JEE Main
IMPORTANT
An open glass tube is immersed in mercury in such a way that a length of extends above the mercury level. The open end of the tube is then closed and sealed and the tube is raised vertically up by additional . What will be length of the air column above mercury in the tube now ?
(Atmospheric pressure = of Hg)
MEDIUM
JEE Main
IMPORTANT
A mass of nitrogen gas is enclosed in a vessel at a temperature, . The amount of heat transferred to the gas, so that velocity of molecules is doubled, is about.
MEDIUM
JEE Main
IMPORTANT
If gas molecules each of mass kg collides with a surface (perpendicular to it) elastically per second over an area with a speed the pressure exerted by the gas molecules will be of the order of:
EASY
JEE Main
IMPORTANT
The number density of molecules of a gas depends on their distance r from the origin as, Then the numer of molecules is proportional to:
MEDIUM
JEE Main
IMPORTANT
A gas molecule of mass at the surface of the earth has kinetic energy equivalent to . If it were to go up straight without colliding with any other molecules, how high would it rise? Assume that the height attained is much less than the radius of the earth. ( is Boltzmann constant)
