MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
Which of the following statement (s) are true about vs graph for a real gas a particular temperature.
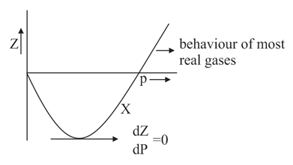

(a)as for most real gases
(b) is - ve as for real gases
(c) at a pressure where repulsive and attractive force are comparable.
(d)At point , intermolecular attractive forces dominating over repulsive forces.
50% studentsanswered this correctly

Important Questions on States of Matter: Gases and Liquids
MEDIUM
JEE Main/Advance
IMPORTANT
One mole of N2O4 gas at 300 K is kept in a closed container at 1 atm. It is heated to 600 K when 20% by mass of N2O4 decomposes to NO2(g). The resultant pressure in the container would be:
EASY
JEE Main/Advance
IMPORTANT
A certain volume of argon gas (Molecular weight) requires to effuse through a hole at a certain pressure and temperature. The same volume of another gas of unknown molecular weight requires to pass through the same hole under the same conditions of temperature and pressure. The molecular weight of the gas is:
HARD
JEE Main/Advance
IMPORTANT
On the surface of the earth at pressure, a balloon filled with gas occupies . This volume is of its maximum stretching capacity. The balloon released left in the air. It starts rising. Calculate the height above which the balloon will burst if the temperature of the atmosphere remains constant and the pressure decreases by for every rise in the height. (nearest integer)
EASY
JEE Main/Advance
IMPORTANT
A chemist has synthesized a greenish yellow gaseous compound of chlorine and oxygen and finds that its density is at and . Then the molecular formula of the compound will be :
HARD
JEE Main/Advance
IMPORTANT
If a gas is expanded at constant temperature:
HARD
JEE Main/Advance
IMPORTANT
Calculate y (ratio of Cp and CV) for triatomic linear gas at high temperature. Assume that the contribution of vibrational degree of freedom is 75% :
MEDIUM
JEE Main/Advance
IMPORTANT
Two closed vessel and of equal volume containing air at pressure and temperature are connected to each other through a narrow open tube. If the temperature of one is now maintained at and other at (where ) then that what will be the final pressure?
HARD
JEE Main/Advance
IMPORTANT
A balloon containing initially, is filled with further air till pressure increases to . The initial diameter of the balloon is and the pressure at each stage is proportion to diameter of the balloon. How many of moles of air added to change the pressure from .
