MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
Which of the following statement is/are correct?
(a)Contributing structures contributes to the resonance hybrid is directly proportional of their energies.
(b)Equivalent contributing structures make the resonance very important.
(c)Contributing structures represent hypothetical molecules having no real existance.
(d)Contributing structures are less stable than the resonance hybrid.
50% studentsanswered this correctly

Important Questions on Organic Chemistry- Some Basic Principles and Techniques
MEDIUM
JEE Main/Advance
IMPORTANT
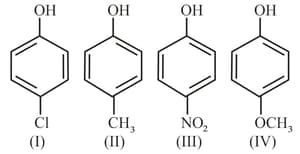
Arrange the following compounds in order of decreasing acidity.
EASY
JEE Main/Advance
IMPORTANT
The order of decreasing basicity in the four halide ions is
MEDIUM
JEE Main/Advance
IMPORTANT
Which of the following is the most stablised carbocation?
EASY
JEE Main/Advance
IMPORTANT
Which one among the following is the least basic
EASY
JEE Main/Advance
IMPORTANT
Which is less basic than benzyl
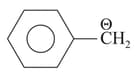 Carbanion?
Carbanion?EASY
JEE Main/Advance
IMPORTANT
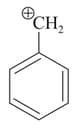
What is the order of stability of the following carbocations ?
MEDIUM
JEE Main/Advance
IMPORTANT
The most stable carbocation is:
MEDIUM
JEE Main/Advance
IMPORTANT
Pyridine is less basic than triethylamine because:
