MEDIUM
Earn 100
Which of the following statement is correct?
(a)-nitrophenol can be separated from -nitrophenol because of intermolecular hydrogen bonding in -nitrophenol.
(b)-nitrophenol can be separated from -nitrophenol because of intermolecular hydrogen bonding in -nitrophenol.
(c)-hydroxybenzoic acid can be separated from -hydrobenzoic acid because of intramolecular hydrogen bonding in -hydroxybenzoic acid.
(d)-hydroxybenzoic acid can be separated from -hydrobenzoic acid because of intermolecular hydrogen bonding in -hydroxybenzoic acid.
50% studentsanswered this correctly
Important Questions on Chemical Bonding and Molecular Structure
EASY
Which one of the following compounds shows the presence of intramolecular hydrogen bond?
MEDIUM
Given:
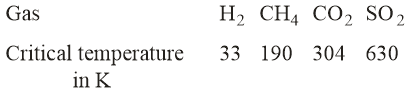
On the basis of data given above, predict which of the following gases shows the least adsorption on a definite amount of charcoal?
MEDIUM
The variation of the boiling points of the hydrogen halides is in the order . Which of the following option explains the higher boiling point of hydrogen fluoride?
HARD
Hydrogen bonding plays a central role in the following phenomena:
MEDIUM
In which of the following solid substance dispersion forces exist?
MEDIUM
The increasing order of the boiling points for the following compounds is:
EASY
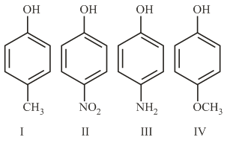
Among the following four aromatic compounds, which one will have the lowest melting point?
MEDIUM
The increasing order of boiling points of the following compounds is :
HARD
The dipole-dipole interaction energy between rotating polar molecules is proportional to, where '' is the distance between polar molecules.
MEDIUM
Dipole - induced dipole interactions are present between which of the following pairs?
MEDIUM
Among the compounds and with molecular formula is having higher boiling point than The possible structures of and are:
MEDIUM
Rare gases are sparingly soluble in water because of
MEDIUM
If the boiling point of is , and the boiling point of will be :
EASY
What is the actual volume occupied by water molecules present in of water?
MEDIUM
Consider the following molecules and statements related to them:
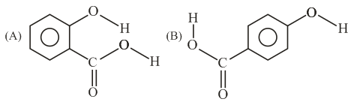
(a) (B) is more likely to be crystaline than (A)
(b) (B) has higher boiling point than (A)
(c) (B) dissolves more readily than (A) in water
Identify the correct option from below:
MEDIUM
Which of the following has the strongest H-bond ?
MEDIUM
Which of the following does not form intra-molecular hydrogen bonding?
EASY
Which effect best explains that -nitrophenol is insoluble in water?
MEDIUM
Give reason: is a liquid and is a gas.
MEDIUM
Match the type of interaction in column with the distance dependence of their interaction energy in column
| A | B |
| (i) ion - ion | (a) |
| (ii) Dipole - dipole | (b) |
| (iii) London dispersion | (c) |
| (d) |

