EASY
AMU-AT (B.Tech.)
IMPORTANT
Earn 100
Which of the following statement is not true?
(a)The pressure of a gas is due to collision of the gas molecules with the walls of the container
(b)The molecular velocity of any gas is proportional to the square root of the absolute temperature
(c)The rate of diffusion of a gas is directly proportional to the density of the gas at constant pressure
(d)Kinetic energy of an ideal gas is directly proportional to the absolute temperature
50% studentsanswered this correctly

Important Questions on States of Matter: Gases and Liquids
MEDIUM
AMU-AT (B.Tech.)
IMPORTANT
Which of the following gases has high Boyle's temperature?
EASY
AMU-AT (B.Tech.)
IMPORTANT
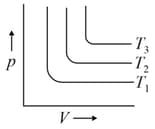
For the given isotherms, which of the following is correct for
EASY
AMU-AT (B.Tech.)
IMPORTANT
If the r.m.s. speed of a gaseous molecule is at a pressure , then what will be the r.m.s. speed at a pressure and constant temperature ?
EASY
AMU-AT (B.Tech.)
IMPORTANT
Which of the following statement is not true?
