MEDIUM
Earn 100
Which of the following statement is true for an azide ion ?
43.04% studentsanswered this correctly
Important Questions on Chemical Bonding and Molecular Structure
EASY
Which of the following sets has Lewis acid behaviour for all the components ?
EASY
Which among the following is an electron deficient compound ?
EASY
The ion that is isoelectronic with is
MEDIUM
is well known but is not.Because,
EASY
Which of the following pair contains lone pair of electrons on the central atom?
HARD
The number of lone pairs present in and respectively, are
EASY
Which of the following molecule does not obey octet rule?
MEDIUM
Which of the following pair of molecules contain odd electron molecule and an expanded octet molecule?
EASY
Valence electrons in the element are and that in element are . Most probable compound formed from and is
MEDIUM
The formal charge on central oxygen atom in ozone is
MEDIUM
The oxide which contains an odd electron at the nitrogen atom is
EASY
Total number of lone pair of electrons in ion is:
MEDIUM
Number of electron deficient molecules among the following and is
MEDIUM
In the given electron dot structure, the formal charge on each nitrogen atom (respectively) from left to right is _______
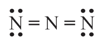
EASY
Amongst and , the number of species with two lone pairs of electrons at central atom is
MEDIUM
The sum of number of lone pairs of electrons present on the central atoms of and is
MEDIUM
Number of lone pairs of electrons in the central atom of and , respectively, are
MEDIUM
Which of the following represents the most stable Lewis dot formula for ?
MEDIUM
In ozone molecule, the formal charge on the central oxygen atom is
EASY
The numbers of lone pair and bond pairs in hydrazine are, respectively:
