MEDIUM
JEE Main
IMPORTANT
Earn 100
Which of the following statements (are) correct?
(a)A metal in its highest oxidation state acts as an oxidant.
(b)
In the reaction,
,
oxygen is an oxidant.
(c)The oxidation number of in is zero.
(d)Copper metal can be oxidised by ions.
50% studentsanswered this correctly

Important Questions on Electrochemistry
MEDIUM
JEE Main
IMPORTANT
Which of the following does not require a salt bridge in them?
EASY
JEE Main
IMPORTANT
How will the of brine ( solution) be affected when it is electrolysed?
MEDIUM
JEE Main
IMPORTANT
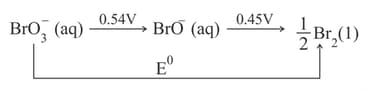
From the standard cell potentials given below, calculate the indicated cell potential ?
MEDIUM
JEE Main
IMPORTANT
Write the Nernst equation for the cell reaction in a Daniel cell. How will the be affected when concentration of ions increase?
HARD
JEE Main
IMPORTANT
How can the reduction potential of an electrode be increased?
MEDIUM
JEE Main
IMPORTANT
The equilibrium constant is related to but not to . Explain why.
EASY
JEE Main
IMPORTANT
What is the free energy change for (a) galvanic cell (b) electrolytic cell?
HARD
JEE Main
IMPORTANT
An excess of solid is added to a solution of ions at . Calculate the concentrations of and ions at equilibrium. Given: and ?
