HARD
Earn 100
Which of the following statements are correct?
1. Order of a reaction can be known from experimental result and not from the stoichiometry of reaction
2. Overall molecularity of a reaction may be determined in a manner similar to overall order of reaction
3. Overall order of reaction,
is
4. Molecularity of a reaction refers to
(i) Molecularity of each of the elementary steps (slow steps) in a multistep reaction
(ii) Molecularity of that particular step in a single step reaction
Select the correct answer by using the codes given below
(a)1, 3 and 4
(b)1, 2 and 3
(c)2, 3 and 4
(d)1, 2 and 4
50% studentsanswered this correctly
Important Questions on Chemical Kinetics
EASY
The kinetic order for the following reaction is:
EASY
HARD
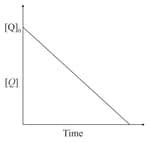
the time taken for reaction of is twice the time taken for reaction of . The concentration of varies with reaction time as shown in the figure.The overall order of the reaction is:
EASY
MEDIUM
EASY
EASY
MEDIUM
EASY
MEDIUM
For the chemical reaction
, the reaction proceeds as follows
(Fast)
, (Slow)
the rate law expression should be given as
MEDIUM
EASY
EASY
MEDIUM
EASY
EASY
EASY

