Which of the following statements are correct?
I. The tangy taste of cola drinks is due to carbonic acid which is an organic acid.
II. Inorganic acids are prepared artificially in industries and are extremely corrosive.
III. Hydrofluoric acid reacts with metals to varying degrees. Therefore, it is stored in glass bottles.
IV. Organic acids are weak acids and are used as food supplements.

Important Questions on Acids, Bases and Salts
Study the given Venn diagram carefully.
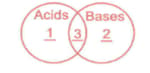
Identify 1,2 and 3.
| 1 | 2 | 3 | |
| (A) | Do not react with metals | React with litmus | Do not react with metals carbonates |
| (B) | Are sour in taste | React with carbonates | Are soapy to touch |
| (C) | Have | Have | Have |
| (D) | React with carbonates to form gas | Turn China rose indicator green | Can be Identified by using indicators |
Ishaan tested the nature of a few common substances with different indicators and recorded his observations as follows:
Substance turned methyl orange yellow.
Substance turned phenolphthalein colourless.
Substance did not have any effect on blue or red litmus.
Substance turned China rose indicator green.
and could be respectively.
A science teacher has arranged the following sets of test tubes as follows.
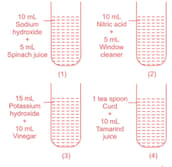
She asked the students to find out the incorrect statement about the above experimental set-up.
Consider the following statements (l-l V) and fill in the blanks by selecting the appropriate option.
I. An antacid such as is used to cure indigestion.
II. Lime water contains , which turns milky with carbon dioxide.
III. Soap solution is basic due to the presence of . It turns the turmeric stain of the shirt to red.
IV. Baking soda is which reacts with vinegar to evolve carbon dioxide gas.
| (A) | Milk of magnesia | Calcium hydroxide | Sodium hydroxide | Sodium hydrogen carbonate |
| (B) | Calcium hydroxide | Milk of magnesia | Sodium hydroxide | Sodium hydrogen carbonate |
| (C) | Sodium hydroxide | Sodium hydrogen carbonate | Calcium hydroxide | Milk of magnesia |
| (D) | Sodium hydrogen carbonate | Calcium hydroxide | Milk of magnesia | Sodium hydroxide |
