MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
Which of the following statements are correct about
(a)The hybridisation of central atom is
(b)Its resonance structure has one single bond and two double bonds.
(c)The average formal charge on each oxygen atom is units.
(d)All bond lengths are equal.
50% studentsanswered this correctly

Important Questions on Chemical Bonding and Molecular Structure
MEDIUM
JEE Main/Advance
IMPORTANT
Which of the following statements are not correct?
MEDIUM
JEE Main/Advance
IMPORTANT
Explain the non linear shape of and non planar shape of using valence shell electron pair repulsion theory.
MEDIUM
JEE Main/Advance
IMPORTANT
Explain the shape of .
MEDIUM
JEE Main/Advance
IMPORTANT
Structures of molecule of two compounds are given below:
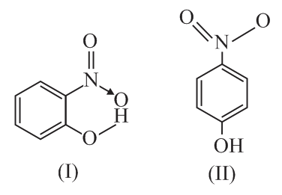
(a) Which of the two compounds will have intermolecular hydrogen bonding and which compound is expected to show intramolecular hydrogen bonding?
(b) The melting point of a compound depends on, among other things, the extent of hydrogen bonding. On this basis, explain which of the above two compounds will show higher melting point.
(c) Solubility of compounds in water depends on power to form hydrogen bonds with water. Which of the above compounds will form hydrogen bond with water easily and be more soluble in it?
EASY
JEE Main/Advance
IMPORTANT
Why does type of overlap given in the following figure not result in bond formation?
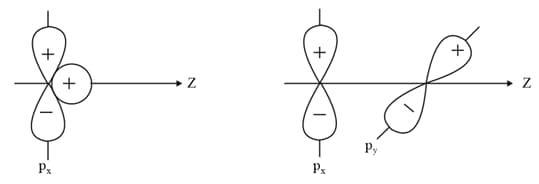
EASY
JEE Main/Advance
IMPORTANT
Explain why is trigonal bipyramidal whereas is square pyramidal.
MEDIUM
JEE Main/Advance
IMPORTANT
In both water and dimethyl ether
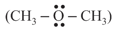 , oxygen atom is central atom, and has the same hybridisation, yet they have different bond angles. Which one has greater bond angle? Give reason.
, oxygen atom is central atom, and has the same hybridisation, yet they have different bond angles. Which one has greater bond angle? Give reason.MEDIUM
JEE Main/Advance
IMPORTANT
Write Lewis structure of the following compounds and show formal charge on each atom.
