EASY
Earn 100
Which of the following statements are correct for carbon compounds?
(i) Most carbon compounds are good conductors of electricity.
(ii) Most carbon compounds are poor conductors of electricity.
(iii) Force of attraction between molecules of carbon compounds is not very strong.
(iv) Force of attraction between molecules of carbon compounds is very strong.
(a)(ii) and (iv)
(b)(ii) and (iii)
(c)(i) and (iv)
(d) (i) and (iii)
50% studentsanswered this correctly
Important Questions on Matter and Its States
HARD
EASY
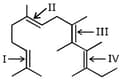
Geometrical isomerism is not possible at the site(s):
MEDIUM
MEDIUM
EASY
EASY
MEDIUM
EASY
The following compounds

are
EASY
MEDIUM
MEDIUM
MEDIUM
MEDIUM
MEDIUM
EASY
EASY
EASY
EASY
MEDIUM
MEDIUM

