Which of the following statements is/are correct?
Important Questions on Organic Chemistry - Some Basic Principles and Techniques
The formula of the purple colour formed in Lassaigne's test for sulphur using sodium nitroprusside is
| Column – | Column - | ||
| A. | Aniline | i. | Red colour with |
| B. | Benzene sulfonic acid | ii. | Violet color with sodium nitroprusside |
| C. | Thiourea | iii. | Blue color with and acidic solution of |
Given below are two statements :
Statement I : In 'Lassaigne's Test', when both nitrogen and sulphur are present in an organic compound, sodium thiocyanate is formed.
Statement II : If both nitrogen and sulphur are present in an organic compound, then the excess of sodium used in sodium fusion will decompose the sodium thiocyanate formed to give and .
In the light of the above statements, choose the most appropriate answer from the options given below
A solution of when treated with gives a prussian blue precipitate due to the formation of
Given below are two statements:
Statement I: Sulphanilic acid gives esterification test for carboxyl group.
Statement II: Sulphanilic acid gives red colour in Lassigne’s test for extra element detection.
In the light of the above statements, choose the most appropriate answer from the options given below:
Match List-I with List-II.
|
List-I Element detected |
List-II Reagent used/Product formed |
||
| Nitrogen | |||
| Sulphur | |||
| Phosphorus | |||
| Halogen | |||
Choose the correct answer from the options given below:
| Item (Compound) | Item (Reagent) | ||
| Lysine | naphthol | ||
| Furfural | Ninhydrin | ||
| Benzyl alcohol | |||
| Styrene | Ceric ammonium nitrate | ||
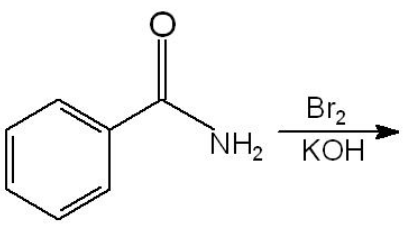
The major product is

