HARD
Chemistry
IMPORTANT
Earn 100
Which of the following statements is/are correct?
(a)Enthalpy of combustion of most species is an exothermic process.
(b)Amongst cyclopropane, cyclobutane and cyclopentane the enthalpy of combustion per group is maximum for cyclopropane.
(c)The enthalpy of formation of acetylene is negative
(d)The entropy of all substances at is positive.
50% studentsanswered this correctly

Important Questions on Thermodynamics
HARD
Chemistry
IMPORTANT
MEDIUM
Chemistry
IMPORTANT
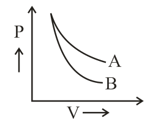
plot for two gases (assuming ideal) during adiabatic processes are given in the figure. Plot and plot should correspond respectively to:
MEDIUM
Chemistry
IMPORTANT
MEDIUM
Chemistry
IMPORTANT
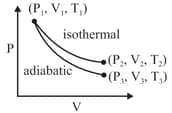
The reversible expansion of an ideal gas under adiabatic and isothermal conditions is shown in the figure. Which of the following statement(s) is (are) correct?
MEDIUM
Chemistry
IMPORTANT
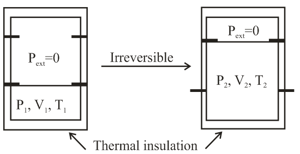
An ideal gas in a thermally insulated vessel at internal pressure volume and absolute temperature expands irreversibly against zero external pressure, as shown in the diagram. The final internal pressure, volume and absolute temperature of the gas are and , respectively. For this expansion
HARD
Chemistry
IMPORTANT
MEDIUM
Chemistry
IMPORTANT
MEDIUM
Chemistry
IMPORTANT
