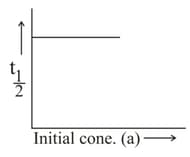
Which of the following statements is/are true?
The order of a reaction can be zero.
The order of a reaction may change, if the experimental conditions are changed.

Important Questions on Chemical Kinetics
Compounds and react with a common reagent with the first-order kinetics in both the cases. If of must react before of has reacted. What is the minimum ratio for their respective rate constants?
(Given: )
The decomposition of at in the gas phase to and is of first order. After at the pressure of falls from to , calculate the rate constant.
The decomposition of at in the gas phase to and is of first-order. After at the pressure of falls from to , calculate the pressure (in atm) of after .
The half-life of the reaction is hours at .
Starting with , what is the mass in of left after ?
The half-life of the reaction is at .
How much time is required to reduce molecules of to molecules?
Which of the following is/are correct for the first order reaction?
(I) 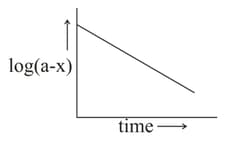
(II) 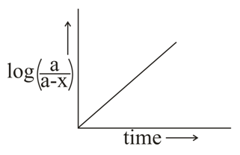
(III)