HARD
10th CBSE
IMPORTANT
Earn 100
Which of the following statements is correct about an aqueous solution of an acid and of a base?
(i) Higher the pH, stronger the acid.
(ii) Higher the pH, weaker the acid.
(iii) Lower the pH, stronger the base.
(iv) Lower the pH, weaker the base.
(iii) Lower the pH, stronger the base.
(iv) Lower the pH, weaker the base.
(a)(i) and (iii)
(b)(ii) and (iii)
(c)(i) and (iv)
(d)(ii) and (iv)
11.11% studentsanswered this correctly

Important Questions on Acids, Bases and Salts
EASY
10th CBSE
IMPORTANT
MEDIUM
10th CBSE
IMPORTANT
EASY
10th CBSE
IMPORTANT
EASY
10th CBSE
IMPORTANT
MEDIUM
10th CBSE
IMPORTANT
HARD
10th CBSE
IMPORTANT
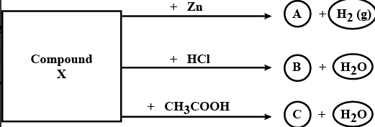
Identify the compound X on the basis of the reactions given below. Also, write the name and chemical formulae of A, B and C.
HARD
10th CBSE
IMPORTANT
HARD
10th CBSE
IMPORTANT
