MEDIUM
Earn 100
Which of the following statements is correct about the given Daniel cell?
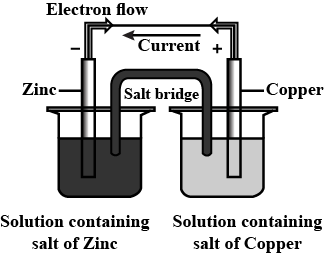

(a)This cell converts the electrical energy liberated during the redox reaction to chemical energy.
(b)This cell has an electrical potential greater than when concentration of
and
ions by unity ()
and
ions by unity ()
(c)In this cell, zinc is acting as anode and copper is acting as cathode.
(d)Redox reaction occurring in this cell is .
50% studentsanswered this correctly
Important Questions on Electrochemistry
MEDIUM
Some materials are given below:
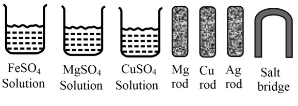
From the given materials, choose the appropriate ones to construct a galvanic cell and draw the diagram of the cell.
(Order of reactivity: )
EASY
MEDIUM
Some materials are given below:
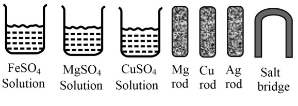
Write the chemical equation fo the reaction taking place at the cathode.
EASY
MEDIUM
Some materials are given below:
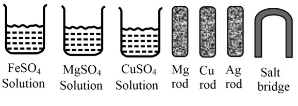
Which is the anode of this cell?
MEDIUM
MEDIUM
EASY
MEDIUM
Depict the galvanic cell in which the reaction takes place. Further show, which of the electrode is negatively charged.
.
EASY
HARD
HARD
The reaction occurs in which of the given galvanic cell?
EASY
MEDIUM
MEDIUM
Represent a cell consisting of | half cell and | half cell and write the cell reaction.
EASY
MEDIUM
EASY
At , the of the galvanic cell mentioned below is
EASY
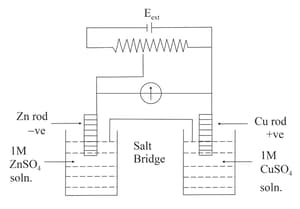
Identify the incorrect statement from the options below for the above cell:

