Which of the following statements is correct for any thermodynamics system?
Important Questions on Thermodynamics
Step It is first compressed adiabatically from volume to
Step then expanded isothermally to volume
Step then expanded adiabatically to volume
Step then compressed isothermally to volume If the efficiency of the above cycle is then is
A number of carnot engines are operated at identical cold reservoir temperatures . However, their hot reservoir temperatures are kept different. A graph of the efficiency of the engines versus hot reservoir temperature is plotted. The correct graphical representation is
An engine operating between the boiling and freezing points of water will have
A. Efficiency more than .
B. Efficiency less than the efficiency of a Carnot engine operating between the same two temperatures.
C. Efficiency equal to .
D. Efficiency less than .
Choose the correct answer from the options given below
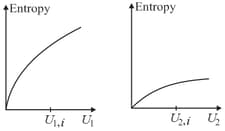
Graphs below show the entropy versus energy of two systems and at constant volume. The initial energies of the systems are indicated by and respectively. Graphs are drawn to the same scale. The systems are then brought into thermal contact with each other. Assume that, at all times the combined energy of the two systems remains constant. Choose the most appropriate option indicating the energies of the two systems and the total entropy after they achieve the equilibrium.
Given the following entropy values at 298 K and 1 atm : The entropy change for the reaction
Work done by an engine is when a heat engine absorbs heat at temperature and heat at temperature. This data:

