EASY
Earn 100
Which of the following statements is incorrect with respect to the solubility of a gas?
(a)Higher the value of Henry's law constant at a given pressure, lower is the solubility of the gas in the liquid
(b)Solubility of a gas in a liquid decreases with increase in temperature and pressure
(c)The dissolution of a gas in a liquid is an exothermic process
(d)All of the above are correct
15.38% studentsanswered this correctly
Important Questions on Solutions
MEDIUM
MEDIUM
EASY
MEDIUM

The gas with the highest value of Henry's law constant is
MEDIUM
Henry's constant (in kbar) for four gases and in water at is given below :
(density of water at ) This table implies that :
EASY
EASY
EASY
MEDIUM
EASY
MEDIUM
HARD
EASY
The oxygen dissolved in water exerts a partial pressure of in the vapour above water. The molar solubility of oxygen in water is ______
(Round off to the Nearest Integer).
[Given : Henry's law constant for Density of water with dissolved oxygen]
EASY
EASY
gas is bubbled through water during a soft drink manufacturing process at . If exerts a partial pressure of then of would dissolve in of water. The value of is _______. (Nearest integer)
(Henry's law constant for at is )
HARD
EASY
From the graph, the value of Henry's constant for the solubility of gas in cyclohexane is
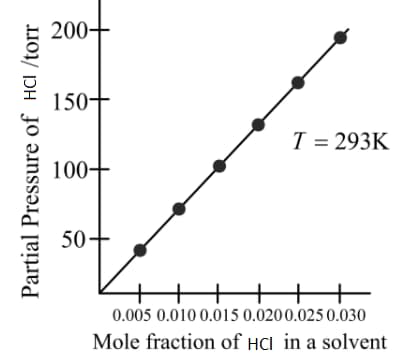
MEDIUM
EASY
EASY

