EASY
Earn 100
Which of the following statements is true for an ion having hybridisation?
(a)All bonds are ionic
(b)H - bonds are situated at the corners of a square
(c)All bonds are co - ordinate covalent
(d)H - atoms are situated at the corners of tetrahedron
50% studentsanswered this correctly
Important Questions on Chemical Bonding and Molecular Structure
EASY
MEDIUM
EASY
(i)
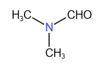 (ii)
(ii) 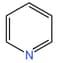
(iii) (iv)
EASY
EASY
EASY
EASY
MEDIUM
MEDIUM
EASY
MEDIUM
EASY
Draw the shape of the following molecule:
EASY
| Column I | Column II |
| a. | (i) T-shape |
| b. | (ii) Pentagonal bipyramidal |
| c. | (iii) Linear |
| d. | (iv) Square – pyramidal |
| (v) Tetrahedral |
MEDIUM
EASY
HARD
EASY
MEDIUM
EASY
EASY
Draw the shape of the following molecule:

