MEDIUM
NEET
IMPORTANT
Earn 100
Which of the graphs shown below does not represent the relationship between the incident light and the electron ejected from the metal surface?
(a)
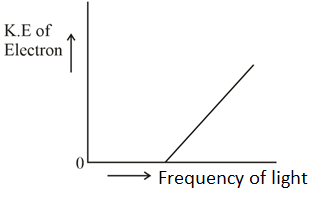
(b)
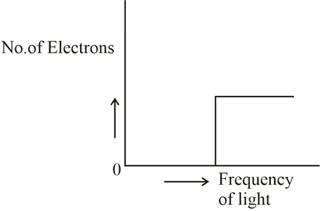
(c)
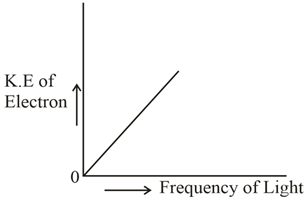
(d)
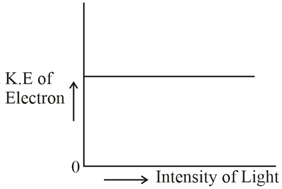
50% studentsanswered this correctly

Important Questions on Structure of Atom
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
