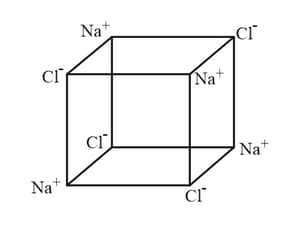
HARD
Upper Secondary-IGCSE
IMPORTANT
Earn 100
Which of the halogen are gases at room temperature and pressure?

Important Questions on Elements and Compounds
HARD
Upper Secondary-IGCSE
IMPORTANT
HARD
Upper Secondary-IGCSE
IMPORTANT
HARD
Upper Secondary-IGCSE
IMPORTANT
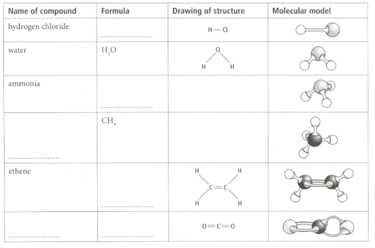
Complete the table by filling in the spaces.
HARD
Upper Secondary-IGCSE
IMPORTANT
Graphite is one of the crystalline forms of carbon. Two of the distinctive properties of graphite are:
- It conducts electricity even though it is a non-metal, and
- It can act as a lubricant even though it has a giant covalent structure.
Give a brief explanation of these properties in light of the structure of graphite.
Graphite as an electrical conductor.
HARD
Upper Secondary-IGCSE
IMPORTANT
Graphite is one of the crystalline forms of carbon. Two of the distinctive properties of graphite are:
- It conducts electricity even though it is a non-metal, and
- It can act as a lubricant even though it has a giant covalent structure.
Give a brief explanation of these properties in light of the structure of graphite.
Graphite are as lubricant.
HARD
Upper Secondary-IGCSE
IMPORTANT
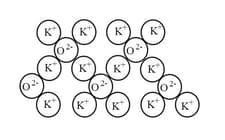
What is the ratio of K+ ions to O2-ions? _____ .
HARD
Upper Secondary-IGCSE
IMPORTANT
The diagram below shows a representation of the structure of an ionic oxide.
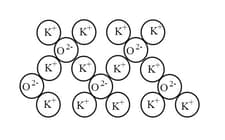
What is the formula of this compound?
HARD
Upper Secondary-IGCSE
IMPORTANT
Extend the structure to the right, by adding four more ions.