MEDIUM
JEE Main
IMPORTANT
Earn 100
Which of the ligands can show linkage isomerism?
(a)
(b)
(c)
(d)
40% studentsanswered this correctly

Important Questions on Coordination Compounds
MEDIUM
JEE Main
IMPORTANT
Which of the following complexes show linkage isomerism?
HARD
JEE Main
IMPORTANT
Draw all the optical isomers for
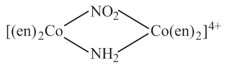
HARD
JEE Main
IMPORTANT
Square planar complexes with coordination number four exhibit geometrical isomerism whereas tetrahedral complexes do not. Why?
HARD
JEE Main
IMPORTANT
Platinum () forms square planar complexes and platinum () gives octahedral complexes. How many geometrical isomers are possible for each of the following complexes? Describe their structures.
HARD
JEE Main
IMPORTANT
Platinum () forms square planar complexes and platinum () gives octahedral complexes. How many geometrical isomers are possible for each of the following complexes? Describe their structures:
HARD
JEE Main
IMPORTANT
Platinum () forms square planar complexes and platinum () gives octahedral complexes. How many geometrical isomers are possible for the following complex? Describe their structures:
HARD
JEE Main
IMPORTANT
Platinum () forms square planar complexes and platinum () gives octahedral complexes. How many geometrical isomers are possible for the following complex? Describe their structures:
HARD
JEE Main
IMPORTANT
Give example of a tetrahedral complex showing optical activity.
