Which of the plants around you need regular watering?
Important Questions on Abdul in the Garden
A solution of glucose containing gram in water was prepared at . What is its osmotic pressure? .
Calculate osmotic pressure of the solution which contains gram sucrose in of solution at .
()
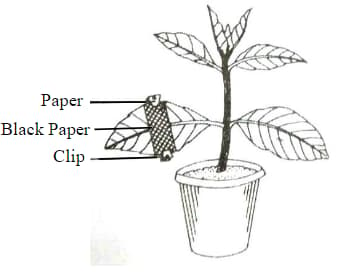
The diagram given below represents an experiment to prove the importance of a factor in photosynthesis. Answer the following question
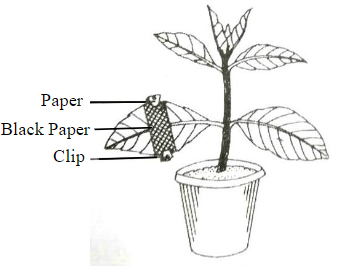
Draw a neat, labelled diagram of an experimental setup to show that oxygen is released during photosynthesis.
Calculate the osmotic pressure of a solution prepared by dissolving of in litres of water at assuming that is completely dissociated. (molecular weight of ).
The diagram given below represents an experiment to prove the importance of a factor in photosynthesis. Answer the following question
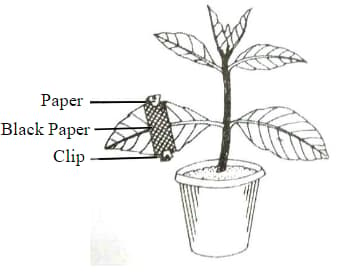
What will you observe in the experimental leaf after the starch test?
The diagram given below represents an experiment to prove the importance of a factor in photosynthesis. Answer the following question
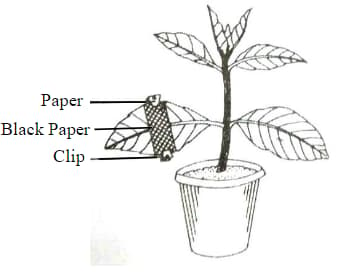
Name the factor studied in this experiment.
The diagram given below represents an experiment to prove the importance of a factor in photosynthesis. Answer the following question
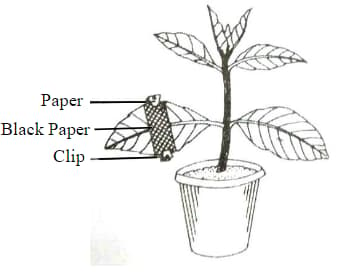
Explain the process of Photosynthesis.
The diagram given below represents an experiment to prove the importance of a factor in photosynthesis. Answer the following question
Give a balanced chemical equation to represent the process of photosynthesis.
