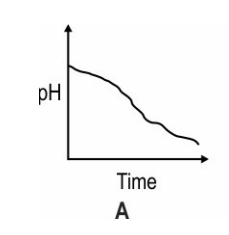
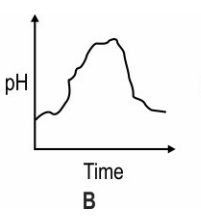
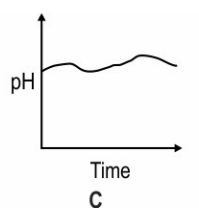
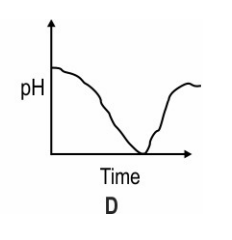
Which of these graphs shows how the pH of milk changes as it forms curd?

Important Questions on Acids, Bases and Salts
The following table lists the pH values of some substances.
| Solution | pH |
| Hydrochloric acid | 1 |
| Milk | 6 |
| Pure water | 7 |
| Baking soda | 9 |
| Sodium hydroxide | 14 |
What would happen to the pH of an acid and a base when each is diluted (pure distilled water is added to it)?
The pH of three solutions is given in the table. Answer the questions that follow.
| Solution | pH |
| P | |
| Q | |
| R |
Which of these solutions could possibly react with zinc metal to produce hydrogen gas?
The pH of three solutions is given in the table. Answer the questions that follow.
| Solution | pH |
| P | |
| Q | |
| R |
Which of these solutions could be formed by the reaction of a metal oxide with water?
| Solution | pH |
| P | |
| Q | |
| R |
Which of these solutions could be the raw material for the industrial manufacture of chlorine?
A remarkable property of acids is that they can 'dissolve' metals. When metals are added to an acid, they disintegrate and disappear into the acid,
State one other common observation when metals 'dissolve' in acids, Explain the reason for this observation.
A remarkable property of acids is that they can 'dissolve' metals. When metals are added to an acid, they disintegrate and disappear into the acid.
If the acid with the 'dissolved' metal is evaporated, can we get the metal back? Why or why not?
A remarkable property of acids is that they can 'dissolve' metals. When metals are added to an acid, they disintegrate and disappear into the acid.
In this question, the word 'dissolve' is used within quotes. This is because it is not actually an example of dissolving. What is the main difference between a metal 'dissolving' in an acid and sugar dissolving in water?
Sunita carried out the following reactions in the laboratory:
- Complete neutralisation of one mole of sodium carbonate with hydrochloric acid
- Complete neutralisation of one mole of sodium bicarbonate with hydrochloric acid
She found that the amount of carbon dioxide formed in both the reactions was the same.
