MEDIUM
Earn 100
Which one is electron deficient compound?
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Chemical Bonding and Structure
EASY
MEDIUM
EASY
HARD
MEDIUM
MEDIUM
In the given electron dot structure, the formal charge on each nitrogen atom (respectively) from left to right is _______
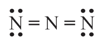
EASY
EASY
EASY
EASY
MEDIUM
EASY
MEDIUM
EASY
MEDIUM
MEDIUM
MEDIUM
MEDIUM
EASY
EASY

