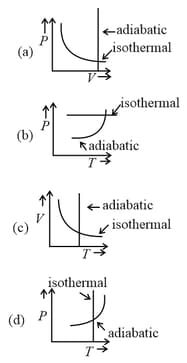
Which one is the correct option for the two different thermodynamic processes ?


Important Questions on Thermodynamics
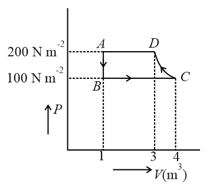
The diagram of a diatomic ideal gas system going under cyclic process as shown in figure. The work done during an adiabatic process is (use :
Match List with List
| List | List | ||
| (a) | Isothermal | (i) | Pressure constant |
| (b) | Isochoric | (ii) | Temperature constant |
| (c) | Adiabatic | (iii) | Volume constant |
| (d) | Isobaric | (iv) | Heat content is constant |
Choose the correct answer from the options given below:
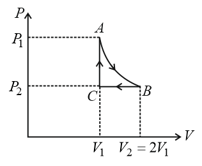
mole of a perfect gas undergoes a cyclic process (see figure) consisting of the following processes.
Isothermal expansion at temperature so that the volume is doubled from to and pressure changes from to
Isobaric compression at pressure to initial volume
Isochoric change leading to change of pressure from to
Total work done in the complete cycle is:
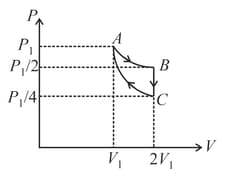
If one mole of an ideal gas at is allowed to expand reversibly and isothermally ( to ) its pressure is reduced to one-half of the original pressure (see figure). This is followed by a constant volume cooling till its pressure is reduced to one-fourth of the initial value Then it is restored to its initial state by a reversible adiabatic compression ( to ). The net workdone by the gas is equal to: