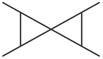
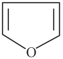
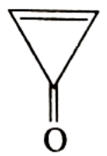
Which one of the following compound is not a planar?
Important Questions on Organic Chemistry
Match List-I with List-II.
| List-I | List-II | ||
| A |
|
I | Spiro compound |
| B |
|
II | Aromatic compound |
| C |
|
III | Non-planar Heterocyclic compound |
| D |
|
IV | Bicyclo compound |
Choose the correct answer from the options given below
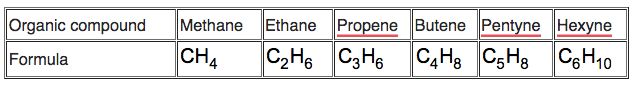
Observe the above table and answer the following question.
Write the formula of hexyne.
Arrange the following according to the instruction given in bracket:
Ethane, methane, ethene, ethyne. (In the increasing order of the molecular weight) []
If the molecular mass of an alkyne is 54, then its molecular formula is _____.
(Enter your correct answer as or )
The structural formula of some organic compounds are given below:
(i)
(ii)
(iii)
(iv)
a. Which of these is an alkane?
b. Write the structural formula of the position isomer of the third compound.
c. Which of the given compounds are functional isomers?
d. Write the structural formula of the chain isomer of the fourth compound.
Petroleum is a mixture of different hydrocarbons.
Which is the hydrocarbon present in liquefied petroleum gas (LPG)?