MEDIUM
10th CBSE
IMPORTANT
Earn 100
Which one of the following correctly represents Sodium oxide?
(a)
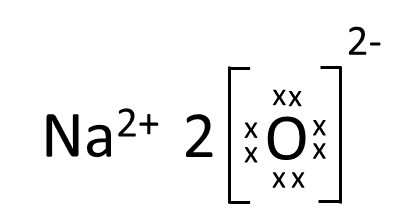
(b)
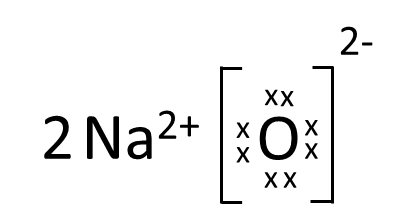
(c)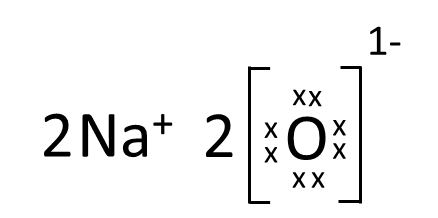
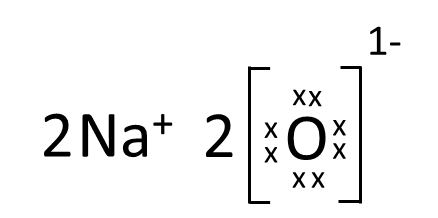
(d)
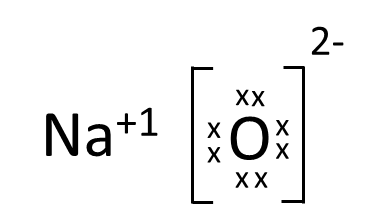
50% studentsanswered this correctly

Important Questions on Metals and Non-metals
EASY
10th CBSE
IMPORTANT
An element with atomic number _____ will form a basic oxide.
EASY
10th CBSE
IMPORTANT
An element has of the electrons filled in the shell as in the shell. The atomic number of is:
HARD
10th CBSE
IMPORTANT
An element ‘M’ with electronic configuration combines separately with anions. Write the chemical formulae of the compounds formed. Predict with the suitable reason the nature of the bond formed by element ‘M’ in general. How will the electrical conductivity of the compounds formed vary with respect to ‘M’?
HARD
10th CBSE
IMPORTANT
A reddish-brown metal ‘X’, when heated in air, gives a black compound ‘Y’, which when heated in the presence of gas gives ‘X’ back. ‘X’ is refined by the process of electrolysis; this refined form of ‘X’ is used in electrical wiring. Identify ‘X’ and ‘Y’. Draw a well-labelled diagram to represent the process of refining ‘X’.
