HARD
JEE Main
IMPORTANT
Earn 100
Which one of the following graphs between molar conductivity versus is correct?
(a)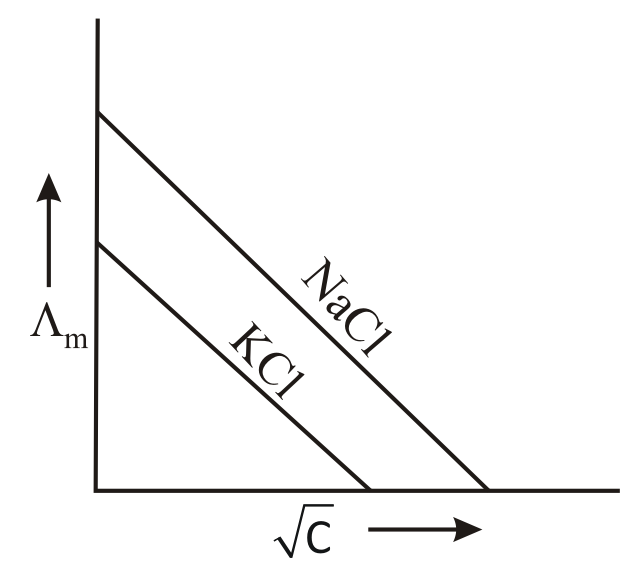

(b)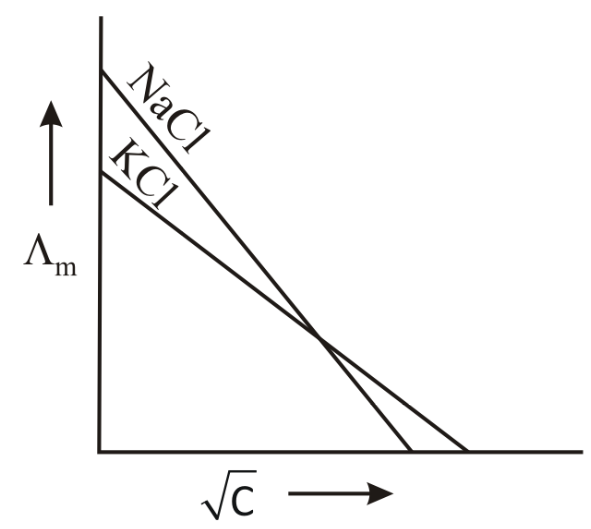

(c)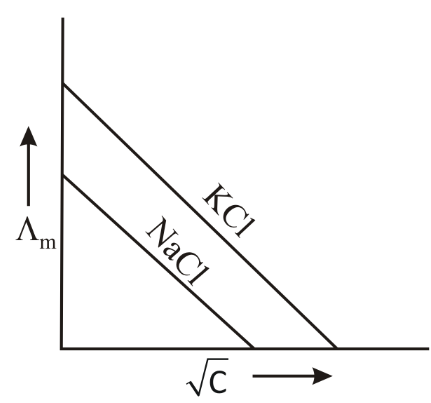

(d)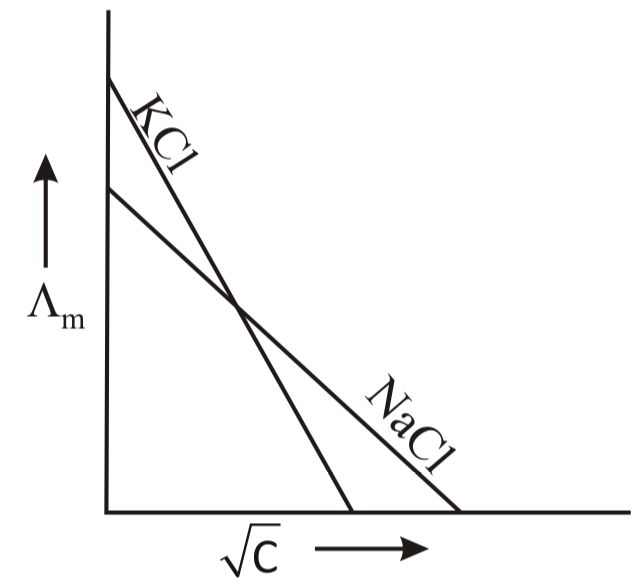

50% studentsanswered this correctly

Important Questions on Electrochemistry
EASY
JEE Main
IMPORTANT
Consider the statements and :
: Conductivity always increases with decreases in the concentration of electrolyte.
: Molar conductivity always increases with decreases in the concentration of electrolyte.
The correct option among the following
MEDIUM
JEE Main
IMPORTANT
Oxidation of succinate ion produces ethylene and carbon dioxide gases. On passing Faraday electricity through an aqueous solution of potassium succinate, what is the total volume of gases (at both cathode and anode) at STP ( and )?
EASY
JEE Main
IMPORTANT
Identify the correct statement:
MEDIUM
JEE Main
IMPORTANT
A variable, the opposite external potential is applied to the cell , of potential . When and , respectively electrons flow from
EASY
JEE Main
IMPORTANT
If the conductivity of mercury at is and the resistance of a cell containing mercury is then the cell constant of the cell is The value of is ______ . (Nearest integer)
MEDIUM
JEE Main
IMPORTANT
The cell potential for the following cell is at . The of the solution is (Nearest integer)
(Given : and )
MEDIUM
JEE Main
IMPORTANT
The resistance of a conductivity cell containing solution at is . If the conductivity of solution at
is , then the cell constant of the conductivity cell is____
MEDIUM
JEE Main
IMPORTANT
In a cell, the following reactions take place
The standard electrode potential for the spontaneous reaction in the cell is . The value of is - (Nearest Integer)
