Which one of the following is NOT an example of heterogeneous equilibrium?
Important Questions on Equilibrium
The reaction
is in equilibrium in a closed vessel at The partial pressure (in atm) of in the reaction vessel is closest to:
[Given: The change in Gibbs energies of formation at and bar for
Gas constant ]
For this equilibrium, the correct statement(s) is/are
The value of for the reaction
The value of for the following reaction is :
When and are compared at It is found that
Using the data provided, find the value of equilibrium constant for the following reaction at and atm pressure.
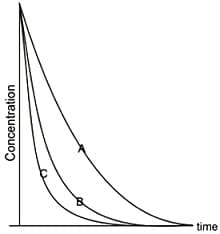
These profiles imply that the decay constants and follow the order
The rate of this reaction is increased by
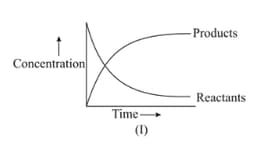
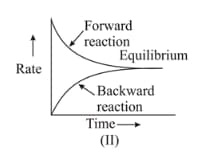
Graph of a reversible process,
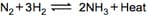
is given. Analyse the graph and answer the following question.
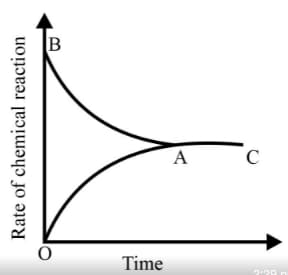
From the given statements, select the correct ones regarding chemical equilibrium.
(i0 The chemical equilibrium is 'static' at the molecular level.
(ii) Both reactants and products co-exist.
(iii) The rates of forward reaction and backward reactions are equal.
(iv) Chemical equilibrium is attained in an open system.
Graph of a reversible process;
is given. Analyse the graph and answer the following question.
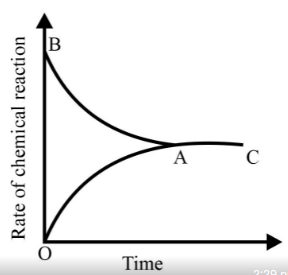
Identify the part of the graph which represents the forward reaction
[ OA, BA, AC]
At equilibrium, the mass of each of the reactants and products remains constant.
At equilibrium, the rate of forward reaction is equal to the rate of backward reaction.
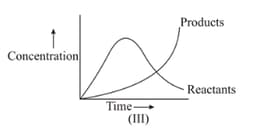
In the reaction , which of the graphs is/are correct?