EASY
Earn 100
Which one of the following is the wrong statement about the liquid?
(a)It has intermolecular force of attraction
(b)Evaporation of liquids increases with the decrease of surface area
(c)It resembles a gas near the critical temperature
(d)It is an intermediate state between gaseous and solid state
80.51% studentsanswered this correctly
Important Questions on States of Matter
HARD
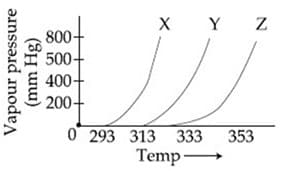
The following inferences are made:
has higher intermolecular interactions compared to
has lower intermolecular interactions compared to
has lower intermolecular interactions compared to
The correct inferences is/are:
MEDIUM
EASY
EASY
EASY
EASY
EASY
EASY
EASY
MEDIUM
EASY
HARD
EASY
MEDIUM
EASY
The portion of the indicator diagram representing the state of matter denotes
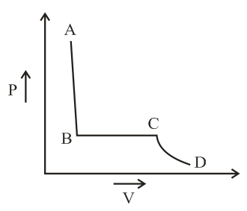
EASY

