EASY
Earn 100
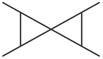
Which one of the following molecules contains only primary and tertiary carbon atoms?
(a)-Dimethylbutane
(b)-Methylpentane
(c)-Dimethylbutane
(d)-Hexane
(e)-Methylhexane
50% studentsanswered this correctly
Important Questions on Hydrocarbons
EASY
HARD
MEDIUM
EASY
EASY
EASY
EASY
EASY
MEDIUM
EASY
MEDIUM
EASY
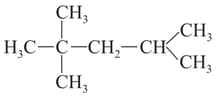
What is the IUPAC name for the following compound?
EASY
Match List-I with List-II.
| List-I | List-II | ||
| A |
|
I | Spiro compound |
| B |
|
II | Aromatic compound |
| C |
|
III | Non-planar Heterocyclic compound |
| D |
|
IV | Bicyclo compound |
Choose the correct answer from the options given below
EASY
EASY
Match the following
| Column I | Column II | ||
| a) | Alkane | (i) | Phenol |
| b) | Alicyclic compound | (ii) | Tropolone |
| c) | Benzenoid aromatic compound | (iii) | Isobutane |
| d) | Non-benzenoid aromatic compound | (iv) | Furan |
| e) | Heterocyclic compound | (v) | Cyclohexene |
EASY
EASY
EASY
EASY
EASY