MEDIUM
Earn 100
Which one of the following molecules is planar?
(a)NF3
(b)NCl3
(c)PH3
(d)BF3
50% studentsanswered this correctly
Important Questions on Chemical Bonding and Molecular Structure
EASY
The compounds containing sp hybridized carbon atom are
(i)
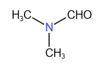 (ii)
(ii) 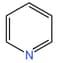
(iii) (iv)
EASY
The shape of by VSEPR theory is:
EASY
The hybridization of the central atom and the shape of ion respectively, are-
EASY
Which of the following pairs of compounds is isoelectronic and isostructural?
MEDIUM
The type of hybridization and no. of lone pair(s) of electron of in , respectively, are:
MEDIUM
hybridization is not displayed by
EASY
Which of the following conversions involves change in both shape and hybridisation?
MEDIUM
The species in which the N atom is a state of sp hybridization is:
EASY
The geometry and magnetic property of respectively, are
MEDIUM
The incorrect geometry is represented by:
EASY
The species having bond angles of is
EASY
In the structure of the number of lone pairs of electrons on the central atom is
EASY
Match the interhalogen compounds of column I with the geometry in column II and assign the correct code.
| Column I | Column II |
| a. | (i) T-shape |
| b. | (ii) Pentagonal bipyramidal |
| c. | (iii) Linear |
| d. | (iv) Square – pyramidal |
| (v) Tetrahedral |
MEDIUM
The group having triangular planar structure is:
EASY
The order of electronegativity of carbon in and hybridized states follows
HARD
The ion that has hybridization for the central atom is:
EASY
The bond angle H-X-H is the greatest in the compound :
MEDIUM
The hybridisation of atomic orbitals of nitrogen in and , respectively are:
EASY
Consider the molecules and . Which of the given statements is false?
EASY
The correct geometry and hybridization for are

