EASY
JEE Main/Advance
IMPORTANT
Earn 100
Which one of the following pairs of molecules will have permanent dipole moments for both members?
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Chemical Bonding and Molecular Structure
EASY
JEE Main/Advance
IMPORTANT
The formation of the oxide ion, , from oxygen atom requires first an exothermic and then an endothermic step as shown in below:
Thus process of formation of in gas phase is unfavourable though is isoelectronic with neon. It is due to the fact that,
MEDIUM
JEE Main/Advance
IMPORTANT
Formation of anion from a neutral atom is favoured by
HARD
JEE Main/Advance
IMPORTANT
In , the bond length is , when is allowed to be treated with , it forms an adduct, , The bond length of in the adduct is :
EASY
JEE Main/Advance
IMPORTANT
The change in hybridization of aluminium when decomposes in the gas phase is :
EASY
JEE Main/Advance
IMPORTANT
The hybridisation of carbon atoms present in the smallest ester are:
HARD
JEE Main/Advance
IMPORTANT
In the following conversion, , the nitrogen atom changes its state of hybridisation from
MEDIUM
JEE Main/Advance
IMPORTANT
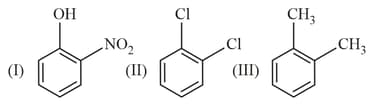
Which one of the following has highest dipole moment?
HARD
JEE Main/Advance
IMPORTANT
The correct order of dipole moment for the following molecules is: