MEDIUM
JEE Main
IMPORTANT
Earn 100
Which one of the following properties is not shown by NO ?
(a)It is diamagnetic in gaseous state
(b)It is a neutral oxide
(c)It combines with oxygen to form nitrogen dioxide
(d)It's bond order is 2.5
33.33% studentsanswered this correctly

Important Questions on Chemical Bonding and Molecular Structure
HARD
JEE Main
IMPORTANT
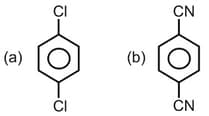
For which of the following molecule significant ?
MEDIUM
JEE Main
IMPORTANT
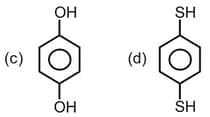
The increasing order of boiling points of the following compounds is :
MEDIUM
JEE Main
IMPORTANT
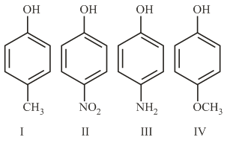
The compound that has the largest bond angle , is :
EASY
JEE Main
IMPORTANT
The potential energy curve for the molecule as a function of internuclear distance is:
MEDIUM
JEE Main
IMPORTANT
The reaction in which the hybridisation of the underlined atom is affected is
EASY
JEE Main
IMPORTANT
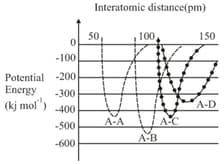
The intermolecular potential energy for the molecules , , and given below suggests that:
MEDIUM
JEE Main
IMPORTANT
The species in which the N atom is a state of sp hybridization is:
HARD
JEE Main
IMPORTANT
According to molecular orbital theory, the number of unpaired electron(s) in is _________ .
